Riboflavin/ja: Difference between revisions
Created page with "===原因=== リボフラビンの欠乏は、通常、他の栄養素、特に他の水溶性ビタミンの欠乏とともにみられる。リボフラビンの欠乏は、一次的なもの(すなわち、通常の食事に含まれるビタミン源が乏しいために起こる)と、二次的なものがあり、腸での吸収に影響を及ぼすような病態の結果として起こることがある。二次的欠乏症は通常、体内で..." Tags: Mobile edit Mobile web edit |
Created page with "1935年、Paul Gyorgyは化学者Richard Kuhnと医師T. Wagner-Jaureggと共同で、B<sub>2</sub>を含まない餌で飼育したラットは体重が増加しないことを報告した。酵母からB<sub>2</sub>を単離したところ、明るい黄緑色の蛍光産物の存在が明らかになり、ラットに与えると正常な成長が回復した。回復した成長は蛍光の強さに正比例した。この観..." Tags: Mobile edit Mobile web edit |
||
| (6 intermediate revisions by the same user not shown) | |||
| Line 338: | Line 338: | ||
リボフラビンの欠乏は、通常、他の栄養素、特に他の水溶性[[vitamin/ja|ビタミン]]の欠乏とともにみられる。リボフラビンの欠乏は、一次的なもの(すなわち、通常の食事に含まれるビタミン源が乏しいために起こる)と、二次的なものがあり、腸での吸収に影響を及ぼすような病態の結果として起こることがある。二次的欠乏症は通常、体内でビタミンが利用されないか、ビタミンの排泄率が増加することによって起こる。欠乏症のリスクを高める食事パターンには、[[veganism/ja|菜食主義]]や低乳製品の[[vegetarianism/ja|ベジタリアン]]などがある。がん、[[heart disease/ja|心臓病]]、[[diabetes/ja|糖尿病]]などの病気は、リボフラビン欠乏症を引き起こしたり、悪化させたりすることがある。 | リボフラビンの欠乏は、通常、他の栄養素、特に他の水溶性[[vitamin/ja|ビタミン]]の欠乏とともにみられる。リボフラビンの欠乏は、一次的なもの(すなわち、通常の食事に含まれるビタミン源が乏しいために起こる)と、二次的なものがあり、腸での吸収に影響を及ぼすような病態の結果として起こることがある。二次的欠乏症は通常、体内でビタミンが利用されないか、ビタミンの排泄率が増加することによって起こる。欠乏症のリスクを高める食事パターンには、[[veganism/ja|菜食主義]]や低乳製品の[[vegetarianism/ja|ベジタリアン]]などがある。がん、[[heart disease/ja|心臓病]]、[[diabetes/ja|糖尿病]]などの病気は、リボフラビン欠乏症を引き起こしたり、悪化させたりすることがある。 | ||
リボフラビンの吸収、輸送、代謝、またはフラボタンパク質による利用を損なうまれな遺伝子異常がある。その一つがリボフラビントランスポーター欠損症であり、以前は[[Brown–Vialetto–Van Laere syndrome/ja|Brown-Vialetto-Van Laere症候群]]として知られていた。[[Transport protein/ja|トランスポータータンパク質]]をコードする遺伝子SLC52A2および[[SLC52A3/ja|SLC52A3]]の変異体である。それぞれRDVT2およびRDVT3に欠陥がある。乳幼児は筋力低下、難聴を含む[[cranial nerve/ja|脳神経]]障害、感覚[[ataxia/ja|運動失調]]を含む感覚症状、摂食障害、呼吸困難を呈する。[[Sensorimotor network/ja|感覚]][[axon/ja|軸索]]性[[neuropathy/ja|ニューロパチー]]および脳神経病理学的所見がある。未治療の場合、リボフラビントランスポーター欠損症の乳児は呼吸困難となり、生後10年以内に死亡する危険性がある。多量のリボフラビンの経口補給による治療が救命につながる。 | |||
その他の先天性代謝異常には、[[glutaric acidemia type 2/ja|グルタル酸血症2型]]のサブセットとしても知られるリボフラビン応答性多発性[[acyl-CoA dehydrogenase/ja|アシル-CoAデヒドロゲナーゼ]]欠損症や、成人では高血圧のリスクと関連している[[methylenetetrahydrofolate reductase/ja|メチレンテトラヒドロ葉酸還元酵素]]のC677T変異体などがある。 | |||
===診断と評価=== | |||
=== | リボフラビン欠乏症が疑われる場合、非特異的症状の症例を確認するためには、リボフラビンの状態を評価することが不可欠である。リボフラビン摂取量が正常な健康成人の総リボフラビン排泄量は1日当たり約120[[:en:microgram|マイクログラム]]であり、1日当たり40マイクログラム未満の排泄は欠乏を示す。リボフラビンの排泄率は加齢とともに減少するが、[[chronic stress/ja|慢性ストレス]]や一部の[[prescription drugs/ja|処方薬]]の使用時には増加する。 | ||
ヒトで用いられる指標は[[erythrocyte/ja|赤血球]][[glutathione reductase/ja|グルタチオン還元酵素]](EGR)、赤血球フラビン濃度、尿中排泄物である。赤血球グルタチオン還元酵素活性係数'' (EGRAC)は、組織の飽和度と長期的なリボフラビン状態の指標となる。結果は活性係数比として表され、培養液にFADを添加した場合と添加しない場合の酵素活性によって決定される。EGRACが1.0~1.2であれば、十分な量のリボフラビンが存在することを示し、1.2~1.4であれば低値、1.4より大きければ欠乏を示す。感度の低い「赤血球フラビン法」では、400nmol/Lを超えると十分量、270nmol/Lを下回ると欠乏とみなされる。 尿中排泄量は、[[creatinine/ja|クレアチニン]]1gあたりのリボフラビンnmolとして表される。低値は50~72nmol/gの範囲内と定義される。欠乏は50nmol/g未満である。食事所要量の決定には、尿中排泄負荷試験が用いられてきた。成人男性では、経口投与量が0.5 mgから1.1 mgに増加すると、尿中リボフラビンの緩やかな直線的増加がみられ、その後の24時間採尿で100マイクログラムに達した。1.1 mgの負荷量を超えると、尿中排泄量は急速に増加し、2.5 mgの投与量では、24時間採尿で尿量は800マイクログラムとなった。 | |||
==歴史== | |||
{{Anchor|History}} | |||
リボフラビン」という名前は、「[[ribose/ja|リボース]]」([[reduction (chemistry)/ja|還元]]型である[[ribitol/ja|リビトール]]が構造の一部を形成する糖)と、酸化型分子に黄色を与える環状部分である「[[Flavin group/ja|フラビン]]」(ラテン語の''flavus''「黄色」に由来する)に由来する。還元型は、酸化型と一緒に代謝され、橙黄色の針状または結晶として現れる。ビタミンが必須栄養素であるという概念よりも前に、最も早く同定が報告されたのは、アレクサンダー・ウィンター・ブライスである。1879年、ブライスは牛乳の乳清に含まれる水溶性の成分を単離し、それを「ラクトクロム」と名付けた。 | |||
1900年代初頭、いくつかの研究所では、ラットの成長維持に不可欠な食品の成分を調査していた。ビタミンBはさらに、B<sub>1</sub>と呼ばれる熱に不安定な物質と、B<sub>2</sub>と呼ばれる熱に不安定な物質の2つの成分があると考えられていた。ビタミンB<sub>2</sub>が[[pellagra/ja|ペラグラ]]の予防に必要な因子であることが仮に確認されたが、それは後に[[Niacin (nutrient)/ja|ナイアシン]](ビタミンB<sub>3</sub>)の欠乏によるものであることが確認された。この混乱は、リボフラビン(B<sub>2</sub>)の欠乏がペラグラに見られるものと似た[[stomatitis/ja|口内炎]]の症状を引き起こすが、広範な末梢の皮膚病変を伴わないという事実によるものであった。このため、ヒトのリボフラビン欠乏症が発見された初期には、この病態は「ペラグラ・シネ・ペラグラ」(ペラグラのないペラグラ)と呼ばれることもあった。 | |||
1935年、[[:en:Paul Gyorgy|Paul Gyorgy]]は化学者[[:en:Richard Kuhn|Richard Kuhn]]と医師T. Wagner-Jaureggと共同で、B<sub>2</sub>を含まない餌で飼育したラットは体重が増加しないことを報告した。酵母からB<sub>2</sub>を単離したところ、明るい黄緑色の蛍光産物の存在が明らかになり、ラットに与えると正常な成長が回復した。回復した成長は蛍光の強さに正比例した。この観察により、研究者たちは1933年に迅速な化学的バイオアッセイ法を開発し、卵白からこの因子を単離し、オボフラビンと名づけた。その後、同じグループが乳清から同様の製剤を単離し、ラクトフラビンと呼んだ。1934年、クーンのグループはこれらのフラビンの化学構造が同一であることを突き止め、名称を「リボフラビン」に決定した。 | |||
1937年頃、リボフラビンは「ビタミンG」とも呼ばれていた。1938年、Richard KuhnはB<sub>2</sub>とB<sub>6</sub>を含むビタミンの研究で[[:en:Nobel Prize in Chemistry|ノーベル化学賞]]を受賞した。1939年、William H. SebrellとRoy E. Butlerが行った臨床試験により、リボフラビンが人間の健康に不可欠であることが確認された。リボフラビンの少ない食事を与えられた女性は口内炎やその他の欠乏症状を呈したが、合成リボフラビンで治療すると症状は回復した。サプリメントを中止すると症状は再発した。 | |||
Latest revision as of 23:40, 19 February 2024
 | |
 化学構造 | |
| Clinical data | |
|---|---|
| Trade names | Many |
| Other names | lactochrome, lactoflavin, vitamin G |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | 口から, intramuscular/ja, intravenous/ja |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 66 to 84 minutes |
| Excretion | 尿 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H20N4O6 |
| Molar mass | 376.369 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
リボフラビン(Riboflavin)は、ビタミンB2としても知られ、食品に含まれるビタミンの一種であり、栄養補助食品として販売されている。2つの主要な補酵素、フラビンモノヌクレオチドとフラビンアデニンジヌクレオチドの形成に必須である。これらの補酵素は、エネルギー代謝、細胞呼吸、抗体産生、および正常な成長と発達に関与している。補酵素はまた、ナイアシン、ビタミンB6、葉酸の代謝にも必要である。リボフラビンは角膜菲薄化を治療するために処方薬されており、経口摂取することで成人の片頭痛の発生率を低下させる可能性がある。
リボフラビン欠乏症はまれで、通常は他のビタミンや栄養素の欠乏を伴う。経口サプリメントまたは注射によって予防または治療することができる。水溶性ビタミンであるリボフラビンは、栄養所要量を超えて摂取しても貯蔵されることはなく、吸収されないか、吸収されてすぐに尿中に排出され、尿が鮮やかな黄色を帯びる。リボフラビンの天然供給源としては、肉、魚、家禽、卵、乳製品、緑黄色野菜、キノコ類、アーモンドなどがある。国によっては、穀類への添加を義務付けているところもある。
リボフラビンは1920年に発見され、1933年に単離され、1935年に初めて合成された。精製された固体の状態では、水溶性の黄橙色の結晶性の粉末である。ビタミンとしての機能に加えて、食品着色料として使用される。生合成は細菌、菌類、植物で行われるが、動物では行われない。リボフラビンの工業的合成は当初化学的プロセスで達成されたが、現在の商業的製造は菌類の菌株や遺伝子組み換え細菌を用いた発酵法に依存している。
定義
ビタミンB2としても知られるリボフラビンは、水溶性のビタミンであり、ビタミンB群の一つである。葉酸やビタミンB6がビタマーとして知られるいくつかの化学的に関連した形で存在するのとは異なり、リボフラビンは1つの化学化合物のみである。それは補酵素フラビンモノヌクレオチド(FMN、リボフラビン-5'-リン酸としても知られる)とフラビンアデニンジヌクレオチド(FAD)の合成における出発化合物である。FADはフラビンの中でもより豊富に存在し、全種類のゲノム(フラボプロテオーム)においてフラビン依存性タンパク質がコードする遺伝子数の75%と結合していると報告されており、ヒトがコードするフラボタンパク質の84%の補酵素として機能している。
リボフラビンは精製された固体の状態では黄橙色の結晶線粉末で、わずかに臭いと苦味がある。水や塩化ナトリウム水溶液などの極性溶媒に溶け、アルコールにもわずかに溶ける。クロロホルム、ベンゼン、アセトンなどの非極性または弱極性有機溶媒には溶けない。リボフラビンは、溶液中または粉末として乾燥保存している間は、光にさらされなければ熱に安定である。加熱して分解すると、一酸化窒素を含む有毒ガスを放出する。
機能
リボフラビンは、2つの主要な補酵素であるFMNとFADの形成に必須である。これらの補酵素はエネルギー代謝、細胞呼吸、抗体産生、成長および発達に関与する。リボフラビンは炭水化物、タンパク質、脂肪の代謝に必須である。FADはトリプトファンからナイアシン(ビタミンB3)への変換に寄与し、ビタミンB6から補酵素ピリドキサール5'-リン酸への変換にはFMNが必要である。リボフラビンは、ホモシステインの正常な循環レベルの維持に関与している。リボフラビン欠乏症では、ホモシステインレベルが上昇し、心血管疾患のリスクが上昇する。
酸化還元反応
酸化還元反応は電子伝達を伴うプロセスである。フラビン補酵素は、酸化型、半還元型、完全還元型の間で変換されるフラビンの能力を利用した1電子または2電子の酸化還元反応を担う、ヒトのおよそ70〜80種類のフラビン酵素(始原菌、細菌、真菌のゲノムにコードされているものを含む、すべての生物全体で数百種類以上)の機能を支えている。FADはまた、内因性の抗酸化物質グルタチオンの形成に不可欠な酵素であるグルタチオン還元酵素の活性にも必要である。
微量栄養素の代謝
リボフラビン、FMN、FADはナイアシン、ビタミンB6、葉酸の代謝に関与する。トリプトファンからのナイアシン含有補酵素であるNADとNADPの合成には、FAD依存性酵素であるキヌレニン3モノオキシゲナーゼが関与している。リボフラビンの欠乏はNADとNADPの産生を減少させ、ナイアシン欠乏を促進する。ビタミンB6からその補酵素であるピリドキサール5'-リン酸合成酵素への変換には、FMNを必要とするピリドキシン5'-リン酸オキシダーゼという酵素が関与する。葉酸代謝に関与する酵素である5,10-メチレンテトラヒドロ葉酸還元酵素は、FMNを必要とする。ホモシステインからアミノ酸であるメチオニンを形成するためにFADを必要とする。
リボフラビンの欠乏は、ヘモグロビンや赤血球の生成に不可欠な栄養ミネラルである鉄の代謝を損なうようである。リボフラビンと鉄の両方が欠乏している人のリボフラビン欠乏を緩和することは、鉄欠乏性貧血を治療するための鉄サプリメントの効果を改善する。
合成
生合成
生合成は細菌、菌類、植物で行われるが、動物では行われない。リボフラビンの生合成前駆体はリブロース5-リン酸とグアノシン3リン酸である。前者はL-3,4-ジヒドロキシ-2-ブタノン-4-リン酸に変換され、後者は一連の反応で5-アミノ-6-(D-リビチルアミノ)ウラシルに変換される。これら2つの化合物は、酵素ルマジン合成酵素の反応EC 2.5.1.78によって触媒される、経路の最後の段階の基質となる。
生合成の最終段階では、2分子の6,7-ジメチル-8-リビチルルマジンが酵素リボフラビン合成酵素によって脱離反応で結合される。これによりリボフラビン1分子と5-アミノ-6-(D-リビチルアミノ)ウラシル1分子が生成される。後者は前の反応にリサイクルされる。
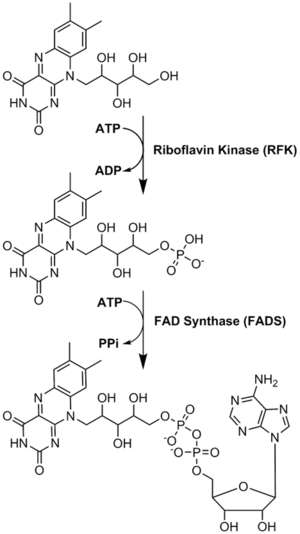
リボフラビンから補酵素への変換が行われる。FMNとFADは、酵素リボフラビンキナーゼとFAD合成酵素が順次作用することによって行われる。
工業合成

リボフラビンの工業的生産には、Ashbya gossypii、Candida famata、Candida flaveriなどの糸状菌や、細菌のCorynebacterium ammoniagenes、枯草菌などを含む様々な微生物が用いられる。リボフラビンの生産量を増やし、抗生物質(アンピシリン)耐性マーカーを導入するために遺伝子組み換えされた枯草菌は、飼料や食品強化のためのリボフラビン生産に商業規模で使用されている。2012年までに、このような発酵プロセスによって年間4,000トン以上が生産された。
高濃度の炭化水素や芳香族化合物が存在すると、一部の細菌はリボフラビンを過剰に産生する。そのような菌の1つがマイクロコッカス・ルテウス(アメリカン・タイプ・カルチャー・コレクション菌株番号ATCC 49442)で、ピリジン上で増殖するとリボフラビンの産生により黄色に発色するが、コハク酸などの他の基質上で増殖すると発色しない。
実験室での合成
リボフラビンの最初の全合成はRichard Kuhnのグループによって行われた。D-リボースを用いた還元的アミノ化によって生成した置換アニリンを、最終段階でアロキサンと縮合させた:
用途
角膜菲薄化の治療
円錐角膜は角膜外反の最も一般的な形態である。角膜が徐々に薄くなる病気であり、この症状は、角膜の硬度を高める角膜コラーゲンクロスリンキングによって治療される。クロスリンキングは、局所リボフラビン溶液を角膜に塗布し、紫外線 A光を照射することで達成される。
片頭痛予防
2012年のガイドラインで米国神経学会は、高用量リボフラビン(400 mg)は「おそらく有効であり、片頭痛予防のために考慮すべきである」と述べており、これは英国国立片頭痛センターも推奨している。2017年のレビューでは、リボフラビンを1日400 mg、少なくとも3ヵ月間毎日摂取することで、成人の片頭痛の頻度が減少する可能性があると報告されている。小児および青年における片頭痛予防または治療のための高用量リボフラビンに関する研究は結論が出ていないため、サプリメントは推奨されていない。
食品着色料
リボフラビンは食品着色料(黄橙色の結晶性粉末)として使用され、ヨーロッパでは食品添加物として使用するためにE101というE番号で指定されている。
食事に関する推奨事項
米国医学アカデミーは、1998年にリボフラビンの推定平均所要量(EAR)と推奨食事許容量(RDA)を更新した。14歳以上の女性および男性のリボフラビンのEARはそれぞれ0.9 mg/日および1.1 mg/日であり、RDAはそれぞれ1.1および1.3 mg/日である。RDAはEARより高く設定されているが、これは必要量が平均より多い人に適切な摂取量を提供するためである。妊娠中のRDAは1.4 mg/日、授乳中の女性のRDAは1.6 mg/日である。生後12ヵ月までの乳児のRDAは0.3~0.4 mg/日であり、1~13歳の子供のRDAは0.5~0.9 mg/日と年齢とともに増加する。安全性に関しては、IOMは十分なエビデンスがある場合、ビタミンとミネラルについて耐容上限摂取量(UL)を設定している。リボフラビンの場合、高用量摂取による有害作用に関するヒトでのデータがないため、ULは設定されていない。EAR、RDA、AI、ULを総称して食事摂取基準(DRI)と呼ぶ。
欧州食品安全機関(EFSA)は、これらの情報をまとめて食事摂取基準値(Dietary Reference Values)と呼び、RDAの代わりに人口摂取基準(Population Reference Intake:PRI)、EARの代わりに平均必要量(Average Requirement)と表記している。AIとULの定義は米国と同じである。15歳以上の女性と男性のPRIは1.6 mg/日とされている。妊娠中のPRIは1.9 mg/日、授乳中の女性のPRIは2.0 mg/日である。1~14歳の小児のPRIは、0.6~1.4 mg/日と年齢とともに増加する。これらのPRIは、米国のRDAよりも高い。EFSAは最大安全摂取量についても検討し、米国国立医学アカデミーと同様、ULを設定するのに十分な情報がないと決定した。
| 推奨される食事許容量 'アメリカ合衆国 | |
| 年齢層(歳) | リボフラビンのRDA(mg/日) |
|---|---|
| 0–6ヶ月 | 0.3* |
| 6–12ヶ月 | 0.4* |
| 1–3 | 0.5 |
| 4–8 | 0.6 |
| 9–13 | 0.9 |
| 女性 14–18 | 1.0 |
| 男性 14–18 | 1.3 |
| 女性 19+ | 1.1 |
| 男性 19+ | 1.3 |
| 妊娠中の女性 | 1.4 |
| 授乳中の女性 | 1.6 |
| * 乳児の適切な摂取量、RDA/RDIはまだ確立されていない | |
| 人口基準摂取量 欧州連合 | |
| 年齢層(歳) | リボフラビンのPRI(mg/日) |
| 7–11ヶ月 | 0.4 |
| 1–3 | 0.6 |
| 4–6 | 0.7 |
| 7–10 | 1.0 |
| 11–14 | 1.4 |
| 15–大人 | 1.6 |
| 妊娠中の女性 | 1.9 |
| 授乳中の女性 | 2.0 |
安全性
ヒトにおいては、過剰摂取によってリボフラビン毒性が生じるという証拠はなく、摂取量が増えるにつれて吸収効率が低下する。 過剰なリボフラビンは腎臓から尿に排泄され、フラビン尿として知られる鮮やかな黄色になる。片頭痛の頻度と重症度の治療に対するリボフラビンの有効性に関する臨床試験では、被験者に1日あたり最大400 mgのリボフラビンを3~12ヵ月間経口投与した。報告された副作用の中には腹痛と下痢があった。
ラベル表示
米国の食品および栄養補助食品の表示目的では、1食あたりの摂取量は1日当たりの摂取量(デイリーバリュー)に対するパーセンテージ(%DV)で表される。リボフラビンの表示目的では、1日当たりの価値の100%は1.7 mgであったが、2016年5月27日付で、RDAと一致させるために1.3 mgに改訂された。新旧の成人一日摂取量の表は、基準一日摂取量に掲載されている。
摂取源=
米国農務省の農業研究局は、数百種類の食品中のリボフラビン含有量を検索できる食品成分データベースを管理している。
|
|
|
小麦を製粉するとリボフラビンが85%失われるため、一部の国では白い小麦粉が強化されている。リボフラビンは、ベビーフード、朝食用シリアル、パスタ、ビタミン強化の食事代替食品にも添加されている。リボフラビンは水への溶解性が低いため、リボフラビン-5'-リン酸(FMN、着色料として使用される場合はE101とも呼ばれる)を使用する必要がある。パンや既製朝食用シリアルの強化は、このビタミンの食事供給に大きく貢献している。遊離リボフラビンは、タンパク質と結合したFMNおよびFADとともに、動物性食品に自然に存在する。牛の乳には主に遊離リボフラビンが含まれるが、FMNとFADの両方が低濃度で存在する。
強化
穀物食品の強化が義務付けられている、あるいは推奨されている国もある。2021年現在、南北アメリカとアフリカ南東部を中心とする56カ国が、小麦粉またはトウモロコシ(コーン)粉にリボフラビンまたはリボフラビン-5'-リン酸ナトリウムを添加することを義務付けている。規定量は1.3~5.75mg/kgである。さらに16カ国が自主的な強化プログラムを実施している。例えば、インド政府は、"マイダ"(白)と"アタ"(全粒粉)の小麦粉に4.0 mg/kgを推奨している。
吸収、代謝、排泄
食事中のリボフラビンの90%以上は、タンパク質と結合したFMNとFADの形で存在する。胃で胃酸にさらされると補酵素が遊離し、その後近位の小腸で酵素的に加水分解されて遊離リボフラビンが放出される。
吸収は迅速な能動輸送系を介して起こり、高濃度ではさらに受動拡散が起こる。胆汁酸塩は取り込みを促進するため、食事と一緒に摂取すると吸収が向上する。成人を対象とした1件の小規模臨床試験では、1回の投与で吸収されるリボフラビンの最大量は27 mgであると報告されている。> 新たに吸収されたリボフラビンの大部分は、1回目の投与で肝臓に取り込まれるため、血漿中のリボフラビンの食後出現は吸収を過小評価する可能性があることを示している。3つのリボフラビントランスポータータンパク質が同定されている: RFVT1は小腸と胎盤に存在し、RFVT2は脳と唾液腺に高発現し、RFVT3は小腸、精巣、前立腺に最も高発現する。これらの輸送タンパク質をコードする遺伝子に変異がある乳児は、リボフラビンの経口投与で治療できる。
リボフラビンは可逆的にFMNに変換され、次にFADに変換される。リボフラビンからFMNへの変換は亜鉛を要求するリボフラビンキナーゼの機能であり、その逆はホスファターゼによって達成される。FMNからFADへはマグネシウムを要求するFAD合成酵素の機能であり、逆はピロホスファターゼによって達成される。FADはそれ自身の生成を抑制する最終生成物であるようだ。
過剰なリボフラビンが小腸で吸収されると、血液から速やかに除去され、尿中に排泄される。尿の色は水分補給状態のバイオマーカーとして用いられ、正常な状態では尿比重および尿浸透圧と相関する。しかし、リボフラビンを必要量を大幅に超えて補給すると、尿が通常よりも黄色く見えるようになる。通常の食事による摂取では、尿中の約3分の2がリボフラビンであり、残りは細胞内での酸化やその他の代謝産物として部分的にヒドロキシメチルリボフラビンに代謝されたものである。消費量が吸収能力を上回ると、リボフラビンは大腸に入り、そこで細菌によって様々な代謝物に異化され、糞便から検出される。吸収されなかったリボフラビンは、大腸のマイクロバイオームに影響を与える可能性があるという推測がある。
欠乏
有病率
リボフラビン欠乏症は、小麦粉やコーンミールの栄養強化プログラムを実施している米国や他の国々ではまれである。年2回実施されている米国人口調査で収集されたデータでは、20歳以上の場合、女性の22%、男性の19%がリボフラビンを含むサプリメント(通常はビタミン・ミネラル複合サプリメント)を摂取していると回答している。サプリメント非使用者の食事からの摂取量は、成人女性で平均1.74 mg/日、男性で2.44 mg/日であった。これらの量は、リボフラビンのRDAである1.1mg/日と1.3mg/日をそれぞれ上回っている。すべての年齢層で、平均して食品からの摂取量がRDAを上回っている。2001-02年の米国の調査では、リボフラビンの推定平均必要量を下回る摂取をしている人は全体の3%未満であったと報告されている。
徴候と症状
リボフラビン欠乏症(リボフラビン症とも呼ばれる)は口内炎を引き起こし、その症状には、唇のひび割れ、口角の炎症(口角口内炎)、喉の痛み、痛みを伴う赤い舌、脱毛が含まれる。目はかゆみ、涙目、充血し、光に敏感になる。リボフラビン欠乏症は貧血を伴う。 リボフラビン欠乏が長期化すると、肝臓および神経系が変性することがある。リボフラビン欠乏は、妊婦の子癇前症のリスクを高める可能性がある。妊娠中のリボフラビン欠乏は、胎児の心臓や四肢の奇形などの先天異常を引き起こす可能性がある。
危険因子
リボフラビン濃度が低くなる危険性があるのは、アルコール中毒者、ベジタリアンのアスリート、菜食主義の実践者などである。母親が肉や乳製品を避けている場合、妊娠中または授乳中の女性とその乳児も危険にさらされる可能性がある。食欲不振や乳糖不耐症はリボフラビン欠乏症のリスクを高める。スポーツ選手や労働者など、肉体的に過酷な生活を送っている人は、より多くのリボフラビン摂取が必要かもしれない。リボフラビンのFADおよびFMNへの変換は、甲状腺機能低下症、副腎不全、およびリボフラビントランスポーター欠損症の人では障害される。
原因
リボフラビンの欠乏は、通常、他の栄養素、特に他の水溶性ビタミンの欠乏とともにみられる。リボフラビンの欠乏は、一次的なもの(すなわち、通常の食事に含まれるビタミン源が乏しいために起こる)と、二次的なものがあり、腸での吸収に影響を及ぼすような病態の結果として起こることがある。二次的欠乏症は通常、体内でビタミンが利用されないか、ビタミンの排泄率が増加することによって起こる。欠乏症のリスクを高める食事パターンには、菜食主義や低乳製品のベジタリアンなどがある。がん、心臓病、糖尿病などの病気は、リボフラビン欠乏症を引き起こしたり、悪化させたりすることがある。
リボフラビンの吸収、輸送、代謝、またはフラボタンパク質による利用を損なうまれな遺伝子異常がある。その一つがリボフラビントランスポーター欠損症であり、以前はBrown-Vialetto-Van Laere症候群として知られていた。トランスポータータンパク質をコードする遺伝子SLC52A2およびSLC52A3の変異体である。それぞれRDVT2およびRDVT3に欠陥がある。乳幼児は筋力低下、難聴を含む脳神経障害、感覚運動失調を含む感覚症状、摂食障害、呼吸困難を呈する。感覚軸索性ニューロパチーおよび脳神経病理学的所見がある。未治療の場合、リボフラビントランスポーター欠損症の乳児は呼吸困難となり、生後10年以内に死亡する危険性がある。多量のリボフラビンの経口補給による治療が救命につながる。
その他の先天性代謝異常には、グルタル酸血症2型のサブセットとしても知られるリボフラビン応答性多発性アシル-CoAデヒドロゲナーゼ欠損症や、成人では高血圧のリスクと関連しているメチレンテトラヒドロ葉酸還元酵素のC677T変異体などがある。
診断と評価
リボフラビン欠乏症が疑われる場合、非特異的症状の症例を確認するためには、リボフラビンの状態を評価することが不可欠である。リボフラビン摂取量が正常な健康成人の総リボフラビン排泄量は1日当たり約120マイクログラムであり、1日当たり40マイクログラム未満の排泄は欠乏を示す。リボフラビンの排泄率は加齢とともに減少するが、慢性ストレスや一部の処方薬の使用時には増加する。
ヒトで用いられる指標は赤血球グルタチオン還元酵素(EGR)、赤血球フラビン濃度、尿中排泄物である。赤血球グルタチオン還元酵素活性係数 (EGRAC)は、組織の飽和度と長期的なリボフラビン状態の指標となる。結果は活性係数比として表され、培養液にFADを添加した場合と添加しない場合の酵素活性によって決定される。EGRACが1.0~1.2であれば、十分な量のリボフラビンが存在することを示し、1.2~1.4であれば低値、1.4より大きければ欠乏を示す。感度の低い「赤血球フラビン法」では、400nmol/Lを超えると十分量、270nmol/Lを下回ると欠乏とみなされる。 尿中排泄量は、クレアチニン1gあたりのリボフラビンnmolとして表される。低値は50~72nmol/gの範囲内と定義される。欠乏は50nmol/g未満である。食事所要量の決定には、尿中排泄負荷試験が用いられてきた。成人男性では、経口投与量が0.5 mgから1.1 mgに増加すると、尿中リボフラビンの緩やかな直線的増加がみられ、その後の24時間採尿で100マイクログラムに達した。1.1 mgの負荷量を超えると、尿中排泄量は急速に増加し、2.5 mgの投与量では、24時間採尿で尿量は800マイクログラムとなった。
歴史
リボフラビン」という名前は、「リボース」(還元型であるリビトールが構造の一部を形成する糖)と、酸化型分子に黄色を与える環状部分である「フラビン」(ラテン語のflavus「黄色」に由来する)に由来する。還元型は、酸化型と一緒に代謝され、橙黄色の針状または結晶として現れる。ビタミンが必須栄養素であるという概念よりも前に、最も早く同定が報告されたのは、アレクサンダー・ウィンター・ブライスである。1879年、ブライスは牛乳の乳清に含まれる水溶性の成分を単離し、それを「ラクトクロム」と名付けた。
1900年代初頭、いくつかの研究所では、ラットの成長維持に不可欠な食品の成分を調査していた。ビタミンBはさらに、B1と呼ばれる熱に不安定な物質と、B2と呼ばれる熱に不安定な物質の2つの成分があると考えられていた。ビタミンB2がペラグラの予防に必要な因子であることが仮に確認されたが、それは後にナイアシン(ビタミンB3)の欠乏によるものであることが確認された。この混乱は、リボフラビン(B2)の欠乏がペラグラに見られるものと似た口内炎の症状を引き起こすが、広範な末梢の皮膚病変を伴わないという事実によるものであった。このため、ヒトのリボフラビン欠乏症が発見された初期には、この病態は「ペラグラ・シネ・ペラグラ」(ペラグラのないペラグラ)と呼ばれることもあった。
1935年、Paul Gyorgyは化学者Richard Kuhnと医師T. Wagner-Jaureggと共同で、B2を含まない餌で飼育したラットは体重が増加しないことを報告した。酵母からB2を単離したところ、明るい黄緑色の蛍光産物の存在が明らかになり、ラットに与えると正常な成長が回復した。回復した成長は蛍光の強さに正比例した。この観察により、研究者たちは1933年に迅速な化学的バイオアッセイ法を開発し、卵白からこの因子を単離し、オボフラビンと名づけた。その後、同じグループが乳清から同様の製剤を単離し、ラクトフラビンと呼んだ。1934年、クーンのグループはこれらのフラビンの化学構造が同一であることを突き止め、名称を「リボフラビン」に決定した。
1937年頃、リボフラビンは「ビタミンG」とも呼ばれていた。1938年、Richard KuhnはB2とB6を含むビタミンの研究でノーベル化学賞を受賞した。1939年、William H. SebrellとRoy E. Butlerが行った臨床試験により、リボフラビンが人間の健康に不可欠であることが確認された。リボフラビンの少ない食事を与えられた女性は口内炎やその他の欠乏症状を呈したが、合成リボフラビンで治療すると症状は回復した。サプリメントを中止すると症状は再発した。