カルシポトリオール
Calcipotriol/ja
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Daivonex, Dovonex, Sorilux |
| Other names | calcipotriene (USAN US) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608018 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Topical administration/ja |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5 to 6% |
| Metabolism | Liver/ja |
| Excretion | 胆道 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
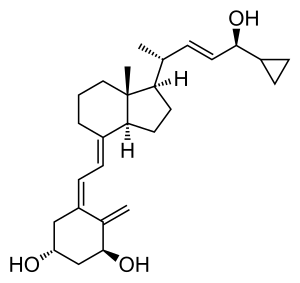
| Formula | C27H40O3 |
| Molar mass | 412.614 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
カルシポトリオール(Calcipotriol)は、ビタミンDの一種であるカルシトリオールの合成誘導体である。 乾癬の治療に用いられる。乾癬性皮膚疾患への長期投与は安全である。
1985年に特許を取得し、1991年に医療用として承認された。米国では「Dovonex」、北米以外では「Daivonex」、ドイツでは「Psorcutan」の商品名で販売されている。
世界保健機関の必須医薬品リストに掲載されている。
カルシポトリオールは、合成コルチコステロイドベタメタゾンジプロピオン酸エステルとの固定用量合剤カルシポトリオール/ベタメタゾンジプロピオン酸エステルとしても利用可能である。この合剤は、斑状乾癬の治療に使用される。
医薬用途
慢性尋常性乾癬はカルシポトリオールの主な医薬品用途である。また、円形脱毛症の治療にも使用され成功を収めている。
Contraindications
Hypersensitivity, use on face, hypercalcaemia, or evidence of vitamin D toxicity are the only contraindications for calcipotriol use.
Cautions include exposure to excessive natural or artificial light, due to the potential for calcipotriol to cause photosensitivity.
Adverse effects
Adverse effects by frequency:
- Very common (> 10% frequency)
- Burning
- Itchiness
- Skin irritation
- Common (1–10% frequency)
- Dermatitis
- Dry skin
- Erythema
- Peeling
- Worsening of psoriasis including facial/scalp
- Rash
- Uncommon (0.1–1% frequency)
- Exacerbation of psoriasis
- Rare (< 0.1% frequency)
- Allergic contact dermatitis
- Hypercalcaemia
- Photosensitivity
- Changes in pigmentation
- Skin atrophy
Interactions
No drug interactions are known.
Pharmacology
Mechanism of action
The efficacy of calcipotriol in the treatment of psoriasis was first noticed by the observation of patients receiving various forms of vitamin D in an osteoporosis study. Unexpectedly, some patients who also had psoriasis experienced dramatic reductions in lesion counts.
The precise mechanism of calcipotriol in remitting psoriasis is not well understood. However, it has been shown to have comparable affinity with calcitriol for the vitamin D receptor (VDR), while being less than 1% as active as the calcitriol in regulating calcium metabolism. The vitamin D receptor belongs to the steroid/thyroid receptor superfamily, and is found on the cells of many different tissues including the thyroid, bone, kidney, and T cells of the immune system. T cells are known to play a role in psoriasis, and it is thought that the binding of calcipotriol to the VDR modulates the T cells gene transcription of cell differentiation and proliferation related genes.
In mouse studies, topical calcipotriol administration to the ear and dorsal skin led to a dose-dependent increase in the production of the epithelial cell-derived cytokine TSLP by keratinocytes, and triggered atopic dermatitis at high concentrations. This upregulation of TSLP production due to calcipotriol application is thought to be mediated through the coactivation of vitamin D receptor/RXRα and vitamin D receptor/RXRβ heterodimers. As psoriasis is typically thought to be partially driven by Th1/Th17 inflammatory cytokines, calcipotriol treatment at appropriate concentrations may alleviate psoriasis symptoms by repressing Th1/Th17 inflammation through TSLP production, which is linked to a Th2 response. However, it is important to note that this has not yet been confirmed.
Pharmacokinetics
After application and systemic uptake, calcipotriol undergoes rapid hepatic metabolism. Calcipotriol is metabolized to MC1046 (the α,β−unsaturated ketone analog), which is subsequently metabolized to its primary metabolite, the saturated ketone analog MC1080. MC1080 is then slowly metabolized to calcitroic acid.
The metabolites of calcipotriol are less potent than the parent compound.
化学
カルシポトリオールは白色からほぼ白色の結晶性化合物である。
外部リンク
- "Calcipotriene". Drug Information Portal. U.S. National Library of Medicine.