SGLT2 inhibitor/ja: Difference between revisions
Created page with "{{Oral hypoglycemics/ja}} {{Sodium-glucose transporter modulators/ja}} {{Portal bar | Medicine}}" Tags: Mobile edit Mobile web edit |
|||
| (2 intermediate revisions by the same user not shown) | |||
| Line 160: | Line 160: | ||
SGLT2阻害薬は循環[[ketone/ja|ケトン体]]濃度を上昇させる。SGLT2阻害薬の[[Cardioprotection/ja|心臓保護]]作用はケトン体濃度の上昇に起因している。 | SGLT2阻害薬は循環[[ketone/ja|ケトン体]]濃度を上昇させる。SGLT2阻害薬の[[Cardioprotection/ja|心臓保護]]作用はケトン体濃度の上昇に起因している。 | ||
グリフロジンは、試験管、前臨床試験および臨床試験において、心臓、肝臓、腎臓の保護作用、抗高脂血症作用、抗[[Atherosclerosis/ja|動脈硬化]]作用、抗[[obesity/ja|肥満]]作用、抗[[Neoplasm/ja|腫瘍]]作用などを示すとされている。 | |||
このクラスの多能性作用は、ナトリウム利尿、血液濃縮、レニン-アンジオテンシン-アルドステロン系の不活性化、ケトン体形成、エネルギー[[homeostasis/ja|恒常性]]の変化、[[glycosuria/ja|糖尿]]、[[lipolysis/ja|脂肪分解]]、[[Anti-inflammatory/ja|抗炎症]]、[[Antioxidant/ja|抗酸化]]などの様々な薬力学的作用に起因している。 | |||
SGLT2阻害薬は、[[:en:NAFLD|NAFLD]]と2型糖尿病の患者を対象とした臨床試験において、また2型糖尿病でない患者を対象とした臨床試験において、肝機能に対する有益な効果を示している。 | |||
==外部リンク== | |||
* {{cite web | title=FDA revises label of diabetes drug canagliflozin | website=U.S. Food and Drug Administration | date=15 January 2016 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet }} | * {{cite web | title=FDA revises label of diabetes drug canagliflozin | website=U.S. Food and Drug Administration | date=15 January 2016 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-label-diabetes-drug-canagliflozin-invokana-invokamet }} | ||
* {{cite web | title=FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) | website=U.S. Food and Drug Administration | date=18 May 2016 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-confirms-increased-risk-leg-and-foot-amputations-diabetes-medicine }} | * {{cite web | title=FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) | website=U.S. Food and Drug Administration | date=18 May 2016 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-confirms-increased-risk-leg-and-foot-amputations-diabetes-medicine }} | ||
| Line 176: | Line 172: | ||
* {{cite web | title=Warning use metformin in certain patients with reduced kidney function | website=U.S. Food and Drug Administration | date=14 November 2017 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain }} | * {{cite web | title=Warning use metformin in certain patients with reduced kidney function | website=U.S. Food and Drug Administration | date=14 November 2017 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-warnings-regarding-use-diabetes-medicine-metformin-certain }} | ||
* {{cite web | title=Warning: infection of genital area with SGLT2 inhibitors for diabetes | website=U.S. Food and Drug Administration | date=7 September 2018 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes }} | * {{cite web | title=Warning: infection of genital area with SGLT2 inhibitors for diabetes | website=U.S. Food and Drug Administration | date=7 September 2018 | url=https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrences-serious-infection-genital-area-sglt2-inhibitors-diabetes }} | ||
{{Oral hypoglycemics/ja}} | |||
{{Oral hypoglycemics}} | {{Sodium-glucose transporter modulators/ja}} | ||
{{Sodium-glucose transporter modulators}} | |||
{{Portal bar | Medicine}} | {{Portal bar | Medicine}} | ||
[[Category:SGLT2 inhibitors| ]] | [[Category:SGLT2 inhibitors| ]] | ||
[[Category:Anti-diabetic drugs]] | [[Category:Anti-diabetic drugs]] | ||
Latest revision as of 09:18, 13 February 2024
| 一般名 | 先発名 | 日本 | 創薬/開発 | 備考 |
|---|---|---|---|---|
| イプラグリフロジン | スーグラ(Sugulat) (PI) (IF) | 販売 | Astellas Pharma |
SGLT2阻害薬は、グリフロジンまたはフロジンとも呼ばれ、腸管粘膜で同様の機能を果たすSGLT1阻害薬とは異なり、ネフロン(腎臓の機能単位)におけるナトリウム-グルコース輸送タンパク質を阻害する医薬品の一種である。この代謝作用の最たるものは、腎臓におけるグルコースの再吸収を阻害し、したがって血糖値を下げることである。これらはナトリウム-グルコース輸送タンパク質2(SGLT2)を阻害することによって作用する。SGLT2阻害薬は2型糖尿病の治療に用いられる。血糖コントロールとは別に、グリフロジンは2型糖尿病の患者において心血管に大きな有益性をもたらすことが示されている。2014年現在、このクラスの医薬品がいくつか承認されているか、開発中である。このクラスのメンバーであるカナグリフロジンに関する研究では、この医薬品は血糖コントロールを高めるだけでなく、体重と収縮期および拡張期血圧を低下させることが明らかにされた。
医薬用途
2022年のADAの糖尿病診療標準では、SGLT2阻害薬は2型糖尿病のファーストライン薬物療法として(通常はメトホルミンと併用する)、特に慢性腎臓病、心血管疾患、心不全を有する患者に使用されている。
SGLT-2阻害薬、GLP-1作動薬、およびDPP-4阻害薬を比較したシステマティックレビューとネットワークメタ解析では、SGLT2阻害薬の使用は、プラセボまたは無治療と比較して死亡の20%減少と関連していることが示された。別の系統的レビューでは、SGLT-2阻害薬が2型糖尿病患者の心腎機能を改善するメカニズムについて論じており、神経緊張の改善における影響を強調している。
13の心血管アウトカム試験を含むメタアナリシスによると、SGLT-2阻害薬は、特に推算糸球体濾過量(eGFR)が60ml/分未満の被験者において、3点MACEのリスクを低下させるが、GLP-1受容体作動薬はeGFRが高い人ほど有益であった。同様に、SGLT-2阻害薬によるリスク低減は、アルブミン尿の割合が高い集団でより大きかったが、GLP-1受容体作動薬ではこの関係は観察されなかった。このことは,腎機能が保たれている患者と低下している患者,あるいは糖尿病性腎症のある患者とない患者で,2つの物質クラスがそれぞれ異なって使用されることを示唆している。
2つのレビューで、SGLT2阻害薬はアテローム性動脈硬化症の主要有害心血管イベント(MACE)患者に有益であると結論されている。そのうちの1つの研究では、MACEを心筋梗塞、脳卒中、心血管死の複合と定義した。
副作用
グリフロジンの副作用としては生殖器感染症が最も多いようである。臨床試験では、真菌感染症、尿路感染症、浸透圧利尿がグリフロジン投与患者で高かった。
2015年5月、FDAはグリフロジンが糖尿病性ケトアシドーシス(DKA、体内でケトン体と呼ばれる血中酸が大量に生成される重篤な状態)のリスクを高める可能性があるとの警告を発した。グルコースの血液循環を減少させることにより、グリフロジンは糖尿病性ケトアシドーシスを引き起こす内因性インスリン分泌の刺激を少なくしたり、外因性インスリンの投与量を少なくしたりする。グリフロジンはケトン体を腎尿細管に吸収させるため、特に優血性DKA(euDKA、血糖が上昇しないDKA)を引き起こすことがある。ケトアシドーシスのリスクが特に高いのは周術期である。SGLT2阻害薬は手術前に中止する必要があるかもしれないが、それは体調が悪くなく、十分な水分補給ができ、通常の食事を摂取できる場合にのみ推奨される。ケトアシドーシスの症状には、吐き気、嘔吐、腹痛、疲労感、呼吸困難などがある。手術後にケトアシドーシスを発症するリスクを軽減するため、FDAはSGLT2阻害糖尿病治療薬の処方情報を変更し、予定されている手術の前に一時的に中止することを推奨することを承認した。カナグリフロジン、ダパグリフロジン、エンパグリフロジンはそれぞれ、手術予定日の少なくとも3日前に、ertugliflozinは少なくとも4日前に中止すべきである。
2015年9月、FDAはカナグリフロジン(インボカーナ)およびカナグリフロジン/メトホルミン(インボカメット)に関連し、骨密度が低下するため骨折のリスクが高まるとして警告を発した。グリフロジンとメトホルミンの併用療法は、スルホニル尿素やインスリンなどの他の2型糖尿病治療薬と比較して、低血糖のリスクを低下させる。
下肢切断のリスク増加はカナグリフロジンと関連しているが、異なるグリフロジンに関連するこのリスクを確認するためにはさらなるデータが必要である。欧州医薬品庁のレビューでは、カナグリフロジン、ダパグリフロジン、エンパグリフロジンを服用している患者では、下肢切断(主に足指に影響)のリスクが増加する可能性があると結論付けられている。
2018年8月、FDAはSGLT2阻害薬使用患者におけるフルニエ壊疽のリスク増加について警告を発した。絶対リスクは非常に低いと考えられている。
FDA有害事象報告システムでは、SGLT2阻害薬に関連した急性腎障害のイベントの増加が報告されているが、臨床試験のデータでは、SGLT-2治療によりそのようなイベントは実際には減少している。
相互作用
2型糖尿病患者の多くは他の多くの医薬品を服用しているため、SGLT2阻害薬の相互作用は重要である。グリフロジンはサイアザイド系薬剤、ループ利尿薬および関連する利尿薬の利尿作用を増強するようであり、脱水および低血圧のリスクを高める可能性がある。治療が併用療法である場合は、低血糖を避けるために抗糖尿病薬の用量を調節することが重要である。例えば、スルホニル尿素との相互作用により、おそらくチトクロームP450に起因すると思われる重篤な低血糖が起こっている。
メンバー
これらはグリフロジンクラスの既知のメンバーである:
- 米国では2023年1月にベキサグリフロジンが商品名Brenzavvyとして承認されている。
- カナグリフロジンは米国で承認された最初のSGLT2阻害薬である。2013年3月に「Invokana」の商品名で承認され、EU全域でも同名で販売されている。
- ダパグリフロジンはフォシーガの製品名で承認され、2012年にEUで承認された最初のSGLT2阻害薬である。米国では2014年1月にFDAからフォシーガの商品名で使用が承認された。英国およびEUでは、インスリンとの併用による1型糖尿病治療薬として初の経口薬である。
- エンパグリフロジンは、ベーリンガーインゲルハイムがJardianceの商品名で2014年8月に米国で承認した。エンパグリフロジンとトホグリフロジンは、グリフロジンの中でSGLT2阻害に対する特異性が最も高い。この2型糖尿病治療薬は、心血管死のリスクを低下させることが示されている。
- 米国では2017年12月にErtugliflozinがSteglatroの商品名で承認された。
- イプラグリフロジンは、日本のアステラス製薬が製造している。日本では2014年1月に承認された。
- ルセオグリフロジンは大正製薬が開発し、日本では「ルセフィ」の商品名で2014年3月に承認された。
- Remogliflozin etabonateは2019年5月にグレンマーク社からインドで最初に商業上市された。
- Sergliflozin etabonateは第II相試験後に販売中止となった。
- Sotagliflozin(Inpefa)はSGLT1/SGLT2デュアル阻害薬であり、心不全または2型糖尿病、慢性腎臓病、その他の心血管危険因子を有する成人において、心血管死、心不全による入院、緊急心不全受診のリスクを低下させることを目的として、2023年5月にFDAから承認された。
- 日本ではサノフィと興和新薬が開発したトホグリフロジンが2014年3月に承認されており、商品名は「アプレウェイ」「デベルザ」である。
- Henagliflozinは、選択的SGLT2阻害薬である。中国で承認されている。
- Janagliflozin.
- Mizagliflozin
- Velagliflozin Proline hydrate
- Enavogliflozin
作用機序
ナトリウムグルコース共輸送体(SGLT)は、主に腎臓に存在し、血液中のグルコースバランスの維持に重要な役割を果たすタンパク質である。SGLT1とSGLT2は、このファミリーの中で最もよく知られている2つのSGLTである。SGLT2は主要な輸送タンパク質であり、糸球体濾過グルコースから循環に戻る再吸収を促進し、腎臓のグルコース再吸収の約90%を担っている。SGLT2は主に腎臓において、近位尿細管の第1分節を裏打ちする上皮細胞に発現している。SGLT2を阻害することにより、グリフロジンは腎臓による糸球体濾液からのグルコースの再吸収を阻害し、血中グルコース濃度を低下させ、尿中グルコースの排泄を促進する(糖尿症)。

細胞レベルでの作用機序はよくわかっていない。有望な利尿促進薬として、この機序を定義する研究が進行中である。しかし、グルコース部位に異なる糖が結合すると、アクセス前庭におけるアグリコンの向きに影響を与えることが示されている。そのため、アグリコンが結合すると阻害剤全体に影響を及ぼす。これらのメカニズムが相乗的な相互作用をもたらす。したがって、糖とアグリコンの両方の構造の変化は、SGLT阻害剤のファーマコフォアにとって極めて重要である。
ダパグリフロジンはSGLT-2阻害薬の一例であり、SGLTに対する競合的で高選択的な阻害薬である。SGLT-2の選択的かつ強力な阻害を介して作用し、その活性は各患者の基礎的な血糖コントロールと腎機能に基づいている。その結果、腎臓でのグルコースの再吸収が低下し、血液循環中のグルコース濃度が高いほどグルコース排泄作用が増加する。したがって、ダパグリフロジンは、他の多くの糖尿病治療薬とは異なり、インスリンの分泌や感受性に依存しない機序で血糖濃度を低下させる。機能的な膵臓のβ細胞は医薬品の活性に必要ではないので、β細胞の機能が低下している患者にも好都合である。
ナトリウムとグルコースは、SGLT-2タンパク質によって近位尿細管の刷子縁膜を越えて尿細管上皮細胞に共輸送される。これは尿細管と細胞の間にナトリウム勾配があるために起こる現象であり、したがってグルコースの二次的な能動輸送を提供する。グルコースはその後、内皮細胞が間質グルコーストランスポータータンパク質に受動的に移行することによって再吸収される。
| SGLT | ヒト組織で発現 |
|---|---|
| SGLT1 | 腸、気管、腎臓、心臓、脳、精巣、前立腺 |
| SGLT2 | 腎臓、脳、肝臓、甲状腺、筋肉、心臓 |
SGLT1とSGLT2の活性比は、発現を定義するのに役立つかもしれない。
薬理学
このクラスの様々な医薬品の消失半減期、バイオアベイラビリティ、タンパク質結合、時間tmaxにおける血中濃度Cmax、およびその他の薬物動態パラメータを表2に示す。これらの医薬品は不活性代謝物として尿中に排泄される。
| 薬物名 | 生物学的利用能 | タンパク質結合 | tmax(時間) | t1/2 (hours) | Cmax | SGLT1に対するSGLT2選択性 |
|---|---|---|---|---|---|---|
| Canagliflozin/ja | 65% (300 mg dose) | 99% | 1–2 | 10.6 (100 mg dose); 13.1 (300 mg dose) | 1096 ng/mL (100 mg dose); 3480 ng/mL (300 mg dose) | 250 fold |
| Dapagliflozin/ja | 78% | 91% | 1–1.5 | 12.9 | 79.6 ng/mL (5 mg dose); 165.0 ng/mL (10 mg dose) | 1200 fold |
| Empagliflozin/ja | 90–97% (mice); 89% (dogs); 31% (rats) | 86.20% | 1.5 | 13.2 (10 mg dose); 13.3h (25 mg dose) | 259nmol/L (10 mg dose); 687nmol/L (25 mg dose) | 2500 fold |
| Ertugliflozin/ja | 70-90% | 95% | 0.5-1.5 | 11-17 | 268 ng/mL (15 mg dose) | 2000 fold |
| イプラグリフロジン (50 mg) | 90% | 96.30% | 1 | 15–16 (50 mg dose) | 975 ng/mL | 360 fold |
| Luseogliflozin/ja | 35.3% (male rats); 58.2% (female rats); 92.7% (male dogs) | 96.0–96.3% | 0.625±0.354 | 9.24±0.928 | 119±27.0 ng/mL | 1650 fold |
| Tofogliflozin/ja (10 mg) | 97.50% | 83% | 0.75 | 6.8 | 489 ng/mL | 2900 fold |
- 'Cmax:' 薬物投与後、薬物が体内で達成する最大血清濃度
- 'tmax: 血漿中濃度が最大になるまでの時間
- t1/2:生物学的半減期
ダパグリフロジンを単回漸増投与(SAD)または反復漸増投与(MAD)した健常人と2型糖尿病患者を対象とした試験では、医薬品の薬物動態プロファイルが確認された。用量依存的な濃度で、半減期は約12~13時間、Tmaxは1~2時間であり、タンパク質結合性であるため、医薬品は速やかに吸収され、腎臓による排泄は最小限である。
ダパグリフロジンの体内動態はBMIや体重に明らかに影響されないため、薬物動態学的知見はBMIの高い患者にも適用できると予想される。ダパグリフロジンの作用機序から予想されるように、ダパグリフロジンは用量依存的に尿中グルコース排泄量を増加させ、単回投与で最大47g/dであった。
いくつかの研究では、ダパグリフロジンはプラセボや他の活性比較薬と比較して統計学的に優れた体重減少に関連することが示されている。これは主に水分よりもカロリーの減少と関連している。
他の抗高血糖糖尿病医薬品とは対照的に、SGLT2阻害薬は糖新生およびケト新生を抑制するのではなく、むしろ亢進させる。SGLT2阻害薬はサーチュイン1(ひいてはPGC-1αとFGF21)を活性化するため、糖尿病の治療に用いられる他の医薬品よりも心臓保護効果が高い。
構造活性相関
グリフロジンの構造活性相関(SAR)は完全には解明されていない。
最も一般的なグリフロジンはダパグリフロジン、エンパグリフロジン、カナグリフロジンである。構造の違いは比較的小さい。一般的な構造には、β位のアノマー炭素に芳香族基を持つグルコース糖が含まれる。グルコース糖部分とβ-異性体のアリール置換基に加えて、アリール基はジアリールメチレン構造からなる。
グリフロジンの合成には一般的に3つのステップがある。最初のステップはアリール置換基の構築であり、次のステップは糖へのアリール部分の導入またはアリール置換基のグルコシル化であり、最後のステップは糖のアリール化アノマー中心の脱保護と修飾である。
フロリジンは最初のタイプのグリフロジンで、SGLT2/SGLT1に対して非選択的であった。これはd-グルコースと芳香族ケトンからなる天然のO-アリール配糖体である。しかし、フロリジンは非常に不安定であり、小腸内のグルコシダーゼによって速やかに分解されるため、糖尿病治療のための経口投与医薬品としては使用できない。この不安定性の問題を克服するために、構造的な改良がなされてきた。C-グルコシドはO-グルコシド誘導体(C-O-C結合の代わりにC-C結合)よりも小腸で安定であるためである。
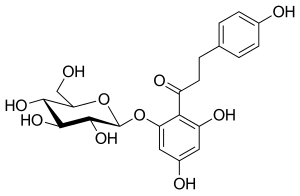
ダパグリフロジンの糖類似体において、β-C系列はα-C系列よりも活性が高いため、阻害活性のためにはβ-配座がC-1であることが重要である。ダパグリフロジンとエンパグリフロジンは、化学構造中に塩素(Cl)原子を含む。Clはハロゲンであり、高い電気陰性度を持っている。この電気陰性度は結合から電子を引き離すため、代謝を低下させる。Cl原子はまた、医薬品のIC50値を低下させるので、医薬品はより優れた活性を持つ。炭素-フッ素結合(C-F)も非常に低い電子密度を持っている。
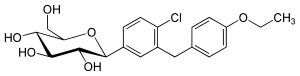
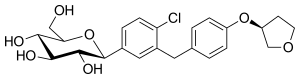
例えば、カナグリフロジンの化学構造において、フッ素原子が芳香環に結合している場合、その化合物はより安定し、代謝が低下する。 エンパグリフロジンにはテトラヒドロフラン環があるが、カナグリフロジンやダパグリフロジンにはない。

グリフロジンの開発において、遠位環は芳香環の代わりにチオフェン環を含んでいた。しかし、市販されているグリフロジンの最終的な化学構造には、このチオフェン環は含まれていない。
歴史
研究
SGLT2阻害薬は循環ケトン体濃度を上昇させる。SGLT2阻害薬の心臓保護作用はケトン体濃度の上昇に起因している。
グリフロジンは、試験管、前臨床試験および臨床試験において、心臓、肝臓、腎臓の保護作用、抗高脂血症作用、抗動脈硬化作用、抗肥満作用、抗腫瘍作用などを示すとされている。 このクラスの多能性作用は、ナトリウム利尿、血液濃縮、レニン-アンジオテンシン-アルドステロン系の不活性化、ケトン体形成、エネルギー恒常性の変化、糖尿、脂肪分解、抗炎症、抗酸化などの様々な薬力学的作用に起因している。
SGLT2阻害薬は、NAFLDと2型糖尿病の患者を対象とした臨床試験において、また2型糖尿病でない患者を対象とした臨床試験において、肝機能に対する有益な効果を示している。
外部リンク
- "FDA revises label of diabetes drug canagliflozin". U.S. Food and Drug Administration. 15 January 2016.
- "FDA Drug Safety Communication: FDA confirms increased risk of leg and foot amputations with the diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR)". U.S. Food and Drug Administration. 18 May 2016.
- "FDA Drug Safety Communication: Interim clinical trial results find increased risk of leg and foot amputations, mostly affecting the toes, with the diabetes medicine canagliflozin (Invokana, Invokamet); FDA to investigate". U.S. Food and Drug Administration. 9 May 2017.
- "Warning use metformin in certain patients with reduced kidney function". U.S. Food and Drug Administration. 14 November 2017.
- "Warning: infection of genital area with SGLT2 inhibitors for diabetes". U.S. Food and Drug Administration. 7 September 2018.