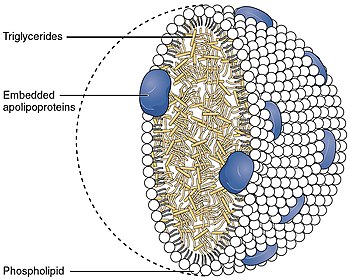
脂質
Lipid/ja

脂質は、脂肪、ワックス、ステロール、脂溶性ビタミン(ビタミンA、D、E、Kなど)、モノグリセリド、ジグリセリド、リン脂質などの有機化合物の広いグループである。脂質の機能には、エネルギーの貯蔵、シグナル伝達、細胞膜の構造成分としての作用などがある。脂質は化粧品や食品、そしてナノテクノロジーにも応用されている。
脂質は広義には疎水性または両親媒性の低分子と定義される。一部の脂質は両親媒性の性質を持つため、小胞、多層/一枚膜リポソーム、または水性環境中の膜などの構造を形成することができる。生物学的脂質は、2つの異なるタイプの生化学的サブユニットまたは「ビルディング・ブロック」から全体的または部分的に生じる: ケトアシル基とイソプレン基である。このアプローチを用いると、脂質は8つのカテゴリーに分けられる: 脂肪アシル類、グリセロ脂質類、グリセロリン脂質類、スフィンゴ脂質類、糖脂質類、ポリケチド類(ケトアシルサブユニットの縮合から誘導される)、およびステロール脂質とプレノール脂質(イソプレンサブユニットの縮合から誘導される)である。
脂質という用語は脂肪の同義語として使われることもあるが、脂肪はトリグリセリドと呼ばれる脂質のサブグループである。脂質はまた、脂肪酸およびその誘導体(トリ、ジ、モノグリセリド、リン脂質を含む)、ならびにコレステロールのような他のステロールを含む代謝産物のような分子を包含する。ヒトをはじめとする哺乳類は、脂質の分解にも合成にも様々な生合成経路を利用しているが、必須脂質の中にはこの方法では作れないものもあり、食事から摂取しなければならないものもある。
歴史
1815年、Henri Braconnotは脂質(graisses)をsuifs(固形油脂)とhuiles(流動油脂)の2つに分類した。1823年、Michel Eugène Chevreulは、油脂、獣脂、ワックス、樹脂、バルサム、揮発油(または精油)を含む、より詳細な分類を開発した。
最初の合成トリグリセリドは1844年にThéophile-Jules Pelouzeによって報告され、彼は濃厚な硫酸の存在下で酪酸をグリセリンで処理してトリブチリンを生成した。数年後、Pelouzeの弟子の一人であったMarcellin Berthelotは、類似の脂肪酸を高温で気体の塩化水素の存在下でグリセリンと反応させることによってトリステアリンとトリパルミチンを合成した。
1827年、ウィリアム・プラウトは、タンパク質(「アルブミン質」)、炭水化物(「サッカリン質」)とともに、脂肪(「油性」消化物)を人間や動物にとって重要な栄養素であると認識した。
1世紀もの間、化学者たちは「脂肪」を脂肪酸とグリセロール(グリセリド)からなる単純な脂質としかみなしていなかったが、後に新しい形態が報告された。セオドア・ゴブリー(1847年)は、哺乳類の脳と鶏卵からリン脂質を発見し、彼はこれを「レシチン類」と呼んだ。トゥディクムは、ヒトの脳からリン脂質(セファリン)、糖脂質(セレブロシド)、スフィンゴ脂質(スフィンゴミエリン)を発見した。
リポイド、リピン、リピド、リピッドという用語は、著者によってさまざまな意味で使われてきた。1912年、ローゼンブルームとギースは「リポイド」を「リピン」に置き換えることを提案した。1920年、Bloorは「リポイド」に新しい分類を導入した:単純リポイド(グリースとワックス)、複合リポイド(リン脂質と糖脂質)、派生リポイド(脂肪酸、アルコール、ステロール)。
ギリシャ語のλίπος「リポス」「脂肪」に語源を持つリピドという言葉は、1923年にフランスの薬理学者ガブリエル・ベルトランによって紹介された。ベルトランは従来の脂肪(グリセリド)だけでなく、複雑な構造を持つ「リポイド」もこの概念に含めた。1923年7月3日のSociété de Chimie Biologique国際委員会の本会議において、lipideという言葉は満場一致で承認された。lipideという単語は、後にその発音('lɪpɪd)からlipidと英語化された。フランス語では、-ideという接尾辞は、古代ギリシャ語の-ίδης(「の息子」または「の子孫」という意味)に由来し、常に(ɪ)と発音される。
1947年、T. P. Hilditchは「単純脂質」をグリースとワックス(真性ワックス、ステロール、アルコール)と定義した。
カテゴリー
脂質は、脂質MAPSコンソーシアムによって以下の8つのカテゴリーに分類されている:
脂肪アシル

。

。
脂肪酸は、脂肪酸合成と呼ばれるプロセスにおいて、アセチル-CoAプライマーとマロニル-CoAまたはメチルマロニル-CoA基との鎖延長によって合成される分子の多様なグループである。脂肪酸はカルボン酸基で終端する炭化水素鎖でできており、この配置により分子は極性を持つ親水性の末端と、水に不溶性である非極性の疎水性の末端を持つ。脂肪酸構造は、生体脂質の最も基本的な分類の一つであり、より構造的に複雑な脂質の構成要素としてよく用いられる。炭素鎖は通常4〜24個の長さで、飽和または不飽和であり、酸素、ハロゲン、窒素、および硫黄を含む官能基が結合している場合がある。脂肪酸が二重結合を含む場合、シスまたはトランスの幾何異性体の可能性があり、これは分子の構成に大きく影響する。シス-二重結合は脂肪酸鎖を曲げる原因となり、鎖中の二重結合が多いほどその効果は増す。植物のチラコイド膜に最も多く存在する脂肪酸アシル鎖である炭素数18のリノレン酸には3つの二重結合があり、低温環境にもかかわらず、これらの膜を非常に流動的にしている。このことは、細胞膜の構造と機能に重要な役割を果たしている。天然に存在する脂肪酸のほとんどはシス型であるが、天然油脂や部分的に水素添加された油脂にはトランス型も存在する。
生物学的に重要な脂肪酸の例としては、主にアラキドン酸とエイコサペンタエン酸から誘導されるエイコサノイドがあり、これにはプロスタグランジン、ロイコトリエン、トロンボキサンが含まれる。ドコサヘキサエン酸もまた、生体系、特に視力に関して重要である。脂肪酸カテゴリーの他の主要な脂質クラスは、脂肪エステルと脂肪アミドである。脂肪エステルには、ワックスエステル、脂肪酸チオエステルコエンザイムA誘導体、脂肪酸チオエステルACP誘導体、脂肪酸カルニチンなどの重要な生化学的中間体が含まれる。脂肪アミドとしては、カンナビノイド神経伝達物質アナンダミドなどのN-アシルエタノールアミンが挙げられる。
グリセロ脂質

グリセロ脂質は一置換、二置換、三置換のグリセロールから構成され、最もよく知られているのはトリグリセリドと呼ばれるグリセロールの脂肪酸トリエステルである。"トリアシルグリセロール"は"トリグリセリド"と同義語として使われることもある。これらの化合物では、グリセロールの3つのヒドロキシル基がそれぞれ、典型的には異なる脂肪酸によってエステル化されている。エネルギー貯蔵物質として機能するため、これらの脂質は動物組織の貯蔵脂肪の大部分を占める。トリグリセリドのエステル結合の加水分解と脂肪組織からのグリセロールと脂肪酸の放出は脂肪を代謝する最初のステップである。
グリセロ脂質の付加的なサブクラスはグリコシルグリセロールで代表され、1つ以上の糖残基がグリコシド結合を介してグリセロールに結合していることが特徴である。このカテゴリーの構造の例としては、植物膜に見られるジガラクトシルジアシルグリセロールや哺乳類の精子細胞のセミノリピドがある。
グリセロリン脂質
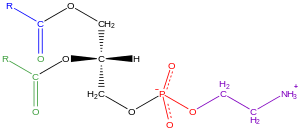
Glycerophospholipids, usually referred to as phospholipids (though sphingomyelins are also classified as phospholipids), are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and cell signaling. Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders. Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.
Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived second messengers. Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria.
Sphingolipids
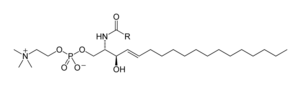
Sphingolipids are a complicated family of compounds that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.
The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phytoceramide phosphoinositols and mannose-containing headgroups. The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides.
Sterols

Sterols, such as cholesterol and its derivatives, are an important component of membrane lipids, along with the glycerophospholipids and sphingomyelins. Other examples of sterols are the bile acids and their conjugates, which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is also used as a biomarker for algal growth. The predominant sterol in fungal cell membranes is ergosterol.
Sterols are steroids in which one of the hydrogen atoms is substituted with a hydroxyl group, at position 3 in the carbon chain. They have in common with steroids the same fused four-ring core structure. Steroids have different biological roles as hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids.:749 The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure.
Prenols

Prenol lipids are synthesized from the five-carbon-unit precursors isopentenyl diphosphate and dimethylallyl diphosphate, which are produced mainly via the mevalonic acid (MVA) pathway. The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin. Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced.
Saccharolipids
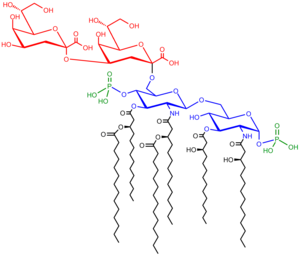
Saccharolipids describe compounds in which fatty acids are linked to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues.
Polyketides
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise many secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity. Many polyketides are cyclic molecules whose backbones are often further modified by glycosylation, methylation, hydroxylation, oxidation, or other processes. Many commonly used antimicrobial, antiparasitic, and anticancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones.
Biological functions
Component of biological membranes
Eukaryotic cells feature the compartmentalized membrane-bound organelles that carry out different biological functions. The glycerophospholipids are the main structural component of biological membranes, as the cellular plasma membrane and the intracellular membranes of organelles; in animal cells, the plasma membrane physically separates the intracellular components from the extracellular environment.Template:Citation needed The glycerophospholipids are amphipathic molecules (containing both hydrophobic and hydrophilic regions) that contain a glycerol core linked to two fatty acid-derived "tails" by ester linkages and to one "head" group by a phosphate ester linkage.Template:Citation needed While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes. In plants and algae, the galactosyldiacylglycerols, and sulfoquinovosyldiacylglycerol, which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are among the most abundant lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.
Plant thylakoid membranes have the largest lipid component of a non-bilayer forming monogalactosyl diglyceride (MGDG), and little phospholipids; despite this unique lipid composition, chloroplast thylakoid membranes have been shown to contain a dynamic lipid-bilayer matrix as revealed by magnetic resonance and electron microscope studies.
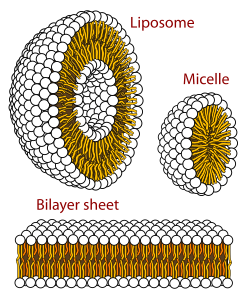
A biological membrane is a form of lamellar phase lipid bilayer. The formation of lipid bilayers is an energetically preferred process when the glycerophospholipids described above are in an aqueous environment. This is known as the hydrophobic effect. In an aqueous system, the polar heads of lipids align towards the polar, aqueous environment, while the hydrophobic tails minimize their contact with water and tend to cluster together, forming a vesicle; depending on the concentration of the lipid, this biophysical interaction may result in the formation of micelles, liposomes, or lipid bilayers. Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behavior. Phase behavior is an area of study within biophysics. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect. When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e., water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment, the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.
The formation of lipids into protocell membranes represents a key step in models of abiogenesis, the origin of life.
Energy storage
Triglycerides, stored in adipose tissue, are a major form of energy storage both in animals and plants. They are a major source of energy in aerobic respiration. The complete oxidation of fatty acids releases about 38 kJ/g (9 kcal/g), compared with only 17 kJ/g (4 kcal/g) for the oxidative breakdown of carbohydrates and proteins. The adipocyte, or fat cell, is designed for continuous synthesis and breakdown of triglycerides in animals, with breakdown controlled mainly by the activation of hormone-sensitive enzyme lipase. Migratory birds that must fly long distances without eating use triglycerides to fuel their flights.
Signaling
Evidence has emerged showing that lipid signaling is a vital part of the cell signaling. Lipid signaling may occur via activation of G protein-coupled or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers. These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization, cell growth, and apoptosis; diacylglycerol and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C; the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity; the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists. Phosphatidylserine lipids are known to be involved in signaling for the phagocytosis of apoptotic cells or pieces of cells. They accomplish this by being exposed to the extracellular face of the cell membrane after the inactivation of flippases which place them exclusively on the cytosolic side and the activation of scramblases, which scramble the orientation of the phospholipids. After this occurs, other cells recognize the phosphatidylserines and phagocytosize the cells or cell fragments exposing them.
Other functions
The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fatty tissues, with a diverse range of functions. Acyl-carnitines are involved in the transport and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extra-cytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for instance, peptidoglycan polymerization in bacteria), and in eukaryotic protein N-glycosylation. Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane. They are believed to activate enzymes involved with oxidative phosphorylation. Lipids also form the basis of steroid hormones.
Metabolism
The major dietary lipids for humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The process of lipid metabolism synthesizes and degrades the lipid stores and produces the structural and functional lipids characteristic of individual tissues.
Biosynthesis
In animals, when there is an oversupply of dietary carbohydrate, the excess carbohydrate is converted to triglycerides. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of fatty acids in the production of triglycerides, a process called lipogenesis. Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acetyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein, while in plant plastids and bacteria separate enzymes perform each step in the pathway. The fatty acids may be subsequently converted to triglycerides that are packaged in lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, the desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly unsaturated fatty acid linoleic acid as well as the triply unsaturated α-linolenic acid cannot be synthesized in mammalian tissues, and are therefore essential fatty acids and must be obtained from the diet.
Triglyceride synthesis takes place in the endoplasmic reticulum by metabolic pathways in which acyl groups in fatty acyl-CoAs are transferred to the hydroxyl groups of glycerol-3-phosphate and diacylglycerol.
Terpenes and isoprenoids, including the carotenoids, are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate. These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA, while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates. One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol. Lanosterol can then be converted into other steroids such as cholesterol and ergosterol.
Degradation
Beta oxidation is the metabolic process by which fatty acids are broken down in the mitochondria or in peroxisomes to generate acetyl-CoA. For the most part, fatty acids are oxidized by a mechanism that is similar to, but not identical with, a reversal of the process of fatty acid synthesis. That is, two-carbon fragments are removed sequentially from the carboxyl end of the acid after steps of dehydrogenation, hydration, and oxidation to form a beta-keto acid, which is split by thiolysis. The acetyl-CoA is then ultimately converted into adenosine triphosphate (ATP), CO2, and H2O using the citric acid cycle and the electron transport chain. Hence the citric acid cycle can start at acetyl-CoA when fat is being broken down for energy if there is little or no glucose available. The energy yield of the complete oxidation of the fatty acid palmitate is 106 ATP. Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation.
Nutrition and health
Most of the fat found in food is in the form of triglycerides, cholesterol, and phospholipids. Some dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, E, and K) and carotenoids. Humans and other mammals have a dietary requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) because they cannot be synthesized from simple precursors in the diet. Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Most vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Alpha-linolenic acid is found in the green leaves of plants and in some seeds, nuts, and legumes (in particular flax, rapeseed, walnut, and soy). Fish oils are particularly rich in the longer-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid. Many studies have shown positive health benefits associated with consumption of omega-3 fatty acids on infant development, cancer, cardiovascular diseases, and various mental illnesses (such as depression, attention-deficit hyperactivity disorder, and dementia).
対照的に、部分水素添加植物油に含まれるようなトランス脂肪酸の摂取が心血管系疾患の危険因子であることは、現在では十分に立証されている。体に良い脂肪が、不適切な調理法によってトランス脂肪酸に変わることがある。
いくつかの研究では、食事から摂取される脂肪の総量が肥満や糖尿病のリスク上昇に関係していることが示唆されている。 49,000人の女性を対象とした8年間の研究であるWomen's Health Initiative Dietary Modification Trial、Nurs' Health Study、Health Professionals Follow-up Studyを含む他の研究では、そのような関連は認められなかった。 これらの研究はいずれも、脂肪からのカロリーの割合とガン、心臓病、体重増加のリスクとの関連を示唆していない。ハーバード大学のT.H.チャン公衆衛生大学院の栄養学科が管理するウェブサイトであるNutrition Sourceは、食事脂肪の影響に関する現在の証拠を要約している。
こちらも参照
- Solid lipid nanoparticle/ja
- Simple lipid/ja
- Emulsion test/ja
- Lipid microdomain/ja
- Membrane lipid/ja
- Lipidomics/ja
- Protein–lipid interaction/ja
- Phenolic lipid/ja, 長い脂肪族鎖とフェノール環からなる天然物の一種で、植物、菌類、バクテリアに存在する。
参考文献
- Bhagavan NV (2002). Medical Biochemistry. San Diego: Harcourt/Academic Press. ISBN 978-0-12-095440-7.
- Devlin TM (1997). Textbook of Biochemistry: With Clinical Correlations (4th ed.). Chichester: John Wiley & Sons. ISBN 978-0-471-17053-2.
- Stryer L, Berg JM, Tymoczko JL (2007). Biochemistry (6th ed.). San Francisco: W.H. Freeman. ISBN 978-0-7167-8724-2.
- van Holde KE, Mathews CK (1996). Biochemistry (2nd ed.). Menlo Park, California: Benjamin/Cummings Pub. Co. ISBN 978-0-8053-3931-4.
外部リンク
入門
- List of lipid-related web sites
- Nature Lipidomics Gateway – Round-up and summaries of recent lipid research
- Lipid Library – General reference on lipid chemistry and biochemistry
- Cyberlipid.org – Resources and history for lipids.
- Molecular Computer Simulations – Modeling of Lipid Membranes
- Lipids, Membranes and Vesicle Trafficking – The Virtual Library of Biochemistry, Molecular Biology and Cell Biology
命名法
データーベース
- LIPID MAPS – Comprehensive lipid and lipid-associated gene/protein databases.
- LipidBank – Japanese database of lipids and related properties, spectral data and references.
一般
- ApolloLipids – Provides dyslipidemia and cardiovascular disease prevention and treatment information as well as continuing medical education programs
- National Lipid Association – Professional medical education organization for health care professionals who seek to prevent morbidity and mortality stemming from dyslipidemias and other cholesterol-related disorders.