High-density lipoprotein/ja: Difference between revisions
Created page with "肝臓はこれらのリポタンパク質をアポリポタンパク質とリン脂質の複合体として合成するが、これはコレステロールを含まない扁平な球状リポタンパク粒子に似ており、最近そのNMR構造が発表された。この複合体はATP結合カセット・トランスポーターA1 (ABCA1)との相互作用によって、細胞内に運ばれたコレステロールを細胞から拾い上げること..." |
|||
| Line 17: | Line 17: | ||
5~17nmの大きさを持つHDLは、[[lipoprotein/ja|リポタンパク質]]粒子の中で最も小さい。[[lipids/ja|脂質]]に対する[[protein/ja|タンパク質]]の割合が最も高いため、最も高密度である。最も豊富な[[apolipoprotein/ja|アポリポ蛋白]]は[[Apolipoprotein A1/ja|アポA-I]]と[[Apolipoprotein A2/ja|アポA-II]]である。まれな遺伝子変異体である[[ApoA-1 Milano/ja|アポA-1 ミラノ]]は、動脈疾患である[[atherosclerosis/ja|アテローム性動脈硬化症]]の予防と回復にはるかに効果的であることが報告されている。 | 5~17nmの大きさを持つHDLは、[[lipoprotein/ja|リポタンパク質]]粒子の中で最も小さい。[[lipids/ja|脂質]]に対する[[protein/ja|タンパク質]]の割合が最も高いため、最も高密度である。最も豊富な[[apolipoprotein/ja|アポリポ蛋白]]は[[Apolipoprotein A1/ja|アポA-I]]と[[Apolipoprotein A2/ja|アポA-II]]である。まれな遺伝子変異体である[[ApoA-1 Milano/ja|アポA-1 ミラノ]]は、動脈疾患である[[atherosclerosis/ja|アテローム性動脈硬化症]]の予防と回復にはるかに効果的であることが報告されている。 | ||
肝臓はこれらのリポタンパク質をアポリポタンパク質とリン脂質の複合体として合成するが、これはコレステロールを含まない扁平な球状リポタンパク粒子に似ており、最近そのNMR構造が発表された。この複合体は[[ABCA1/ja|ATP結合カセット・トランスポーターA1 (ABCA1)]]との相互作用によって、細胞内に運ばれたコレステロールを細胞から拾い上げることができる。[[lecithin-cholesterol acyltransferase/ja|レシチン-コレステロールアシルトランスフェラーゼ]](LCAT)と呼ばれる[[Blood plasma/ja|血漿]]酵素は遊離コレステロールをコレステリルエステル(コレステロールのより疎水性の形)に変換し、リポタンパク質粒子のコアに封じ込められ、最終的に新しく合成されたHDLは球状になる。HDL粒子は血液中を循環するにつれて大きくなり、[[ABCG1/ja|ABCG1]]トランスポーターや[[PLTP/ja|リン脂質輸送タンパク質(PLTP)]]との相互作用などにより、細胞や他のリポタンパク質からより多くのコレステロールやリン脂質分子を取り込む。 | |||
<div lang="en" dir="ltr" class="mw-content-ltr"> | <div lang="en" dir="ltr" class="mw-content-ltr"> | ||
Revision as of 19:51, 9 March 2024
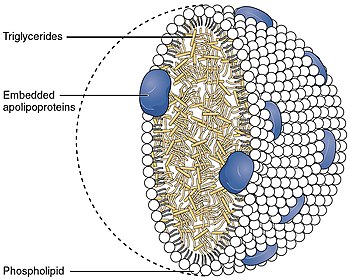
高密度リポ蛋白質(HDL)は、リポ蛋白質の5大グループの1つである。リポ蛋白質は複数の蛋白質からなる複雑な粒子であり、細胞外の水分の中で体内の全ての脂肪分子(脂質)を輸送する。通常、粒子あたり80~100個のタンパク質で構成されている(1個、2個または3個のApoAによって組織されている)。HDL粒子は血液中を循環する間に大きくなり、より多くの脂肪分子を凝集させ、1粒子あたり最大数百の脂肪分子を輸送する。
概要
リポ蛋白質は密度/サイズ(逆相関関係)により5つのサブグループに分けられ、これは機能および心血管イベントの発生率とも相関している。脂肪分子を細胞に運ぶ大きなリポタンパク質粒子とは異なり、HDL粒子は細胞から脂肪分子を除去する。運ばれる脂質にはコレステロール、リン脂質、トリグリセリドがあり、それぞれの量は変動する。
HDL粒子の濃度を高めることは、動脈壁内のアテローム性動脈硬化症の蓄積を減少させ、突然のプラーク破裂、心血管疾患、脳卒中、その他の血管疾患のリスクを減少させることと関連している。HDL粒子は一般に「善玉コレステロール」と呼ばれるが、これは動脈壁から脂肪分子を運び出し、マクロファージの蓄積を抑え、動脈硬化の予防や退縮に役立つからである。しかし、最近の研究では、非常に高濃度のHDL粒子は、特に高血圧患者において、死亡リスクの増加や心血管リスクの増加と関連する可能性があることが示されている。
検査
HDLとLDL(低比重リポ蛋白)蛋白粒子を直接測定するのはコストが高いため、血液検査では代用値であるHDL-C、すなわちApoA-1/HDL粒子に関連するコレステロールを測定するのが一般的である。健康な人では、血中コレステロールの約30%が他の脂肪とともにHDLによって運ばれる。これはしばしば、低比重リポ蛋白粒子LDL内で運ばれると推定されるコレステロールの量と対比され、LDL-Cと呼ばれる。HDL粒子は動脈壁アテローム内を含む細胞から脂肪とコレステロールを除去し、排泄または再利用のために肝臓に戻す。したがって、HDL粒子内に運ばれるコレステロール(HDL-C)は、(LDL粒子内のコレステロールと同じであるにもかかわらず)「善玉コレステロール」と呼ばれることがある。HDL-Cの値が高い人は心血管系疾患の問題が少ない傾向があるが、HDL-Cコレステロール値が低い人(特に40 mg/dL未満または約1 mmol/L未満)は心臓病の罹患率が高くなる。健康な人の場合、本来のHDL値が高いほど心血管疾患のリスクが低下する。
HDLを差し引いた残りの血清コレステロールが非HDLコレステロールである。アテロームを引き起こす可能性のあるこれらの他の成分の濃度は、非HDL-Cとして知られている。非HDL-Cは、より良い予測因子であることが示されており、計算も容易であるため、現在では二次的なマーカーとしてLDL-Cよりも好まれている。
構造と機能
5~17nmの大きさを持つHDLは、リポタンパク質粒子の中で最も小さい。脂質に対するタンパク質の割合が最も高いため、最も高密度である。最も豊富なアポリポ蛋白はアポA-IとアポA-IIである。まれな遺伝子変異体であるアポA-1 ミラノは、動脈疾患であるアテローム性動脈硬化症の予防と回復にはるかに効果的であることが報告されている。
肝臓はこれらのリポタンパク質をアポリポタンパク質とリン脂質の複合体として合成するが、これはコレステロールを含まない扁平な球状リポタンパク粒子に似ており、最近そのNMR構造が発表された。この複合体はATP結合カセット・トランスポーターA1 (ABCA1)との相互作用によって、細胞内に運ばれたコレステロールを細胞から拾い上げることができる。レシチン-コレステロールアシルトランスフェラーゼ(LCAT)と呼ばれる血漿酵素は遊離コレステロールをコレステリルエステル(コレステロールのより疎水性の形)に変換し、リポタンパク質粒子のコアに封じ込められ、最終的に新しく合成されたHDLは球状になる。HDL粒子は血液中を循環するにつれて大きくなり、ABCG1トランスポーターやリン脂質輸送タンパク質(PLTP)との相互作用などにより、細胞や他のリポタンパク質からより多くのコレステロールやリン脂質分子を取り込む。
HDL transports cholesterol mostly to the liver or steroidogenic organs such as adrenals, ovary, and testes by both direct and indirect pathways. HDL is removed by HDL receptors such as scavenger receptor BI (SR-BI), which mediate the selective uptake of cholesterol from HDL. In humans, probably the most relevant pathway is the indirect one, which is mediated by cholesteryl ester transfer protein (CETP). This protein exchanges triglycerides of VLDL against cholesteryl esters of HDL. As the result, VLDLs are processed to LDL, which are removed from the circulation by the LDL receptor pathway. The triglycerides are not stable in HDL, but are degraded by hepatic lipase so that, finally, small HDL particles are left, which restart the uptake of cholesterol from cells.
The cholesterol delivered to the liver is excreted into the bile and, hence, intestine either directly or indirectly after conversion into bile acids. Delivery of HDL cholesterol to adrenals, ovaries, and testes is important for the synthesis of steroid hormones.
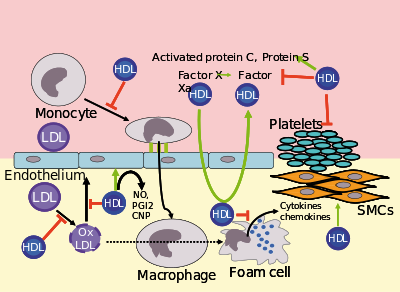
Several steps in the metabolism of HDL can participate in the transport of cholesterol from lipid-laden macrophages of atherosclerotic arteries, termed foam cells, to the liver for secretion into the bile. This pathway has been termed reverse cholesterol transport and is considered as the classical protective function of HDL toward atherosclerosis.
HDL carries many lipid and protein species, several of which have very low concentrations but are biologically very active. For example, HDL and its protein and lipid constituents help to inhibit oxidation, inflammation, activation of the endothelium, coagulation, and platelet aggregation. All these properties may contribute to the ability of HDL to protect from atherosclerosis, and it is not yet known which are the most important. In addition, a small subfraction of HDL lends protection against the protozoan parasite Trypanosoma brucei brucei. This HDL subfraction, termed trypanosome lytic factor (TLF), contains specialized proteins that, while very active, are unique to the TLF molecule.
In the stress response, serum amyloid A, which is one of the acute-phase proteins and an apolipoprotein, is under the stimulation of cytokines (interleukin 1, interleukin 6), and cortisol produced in the adrenal cortex and carried to the damaged tissue incorporated into HDL particles. At the inflammation site, it attracts and activates leukocytes. In chronic inflammations, its deposition in the tissues manifests itself as amyloidosis.
It has been postulated that the concentration of large HDL particles more accurately reflects protective action, as opposed to the concentration of total HDL particles. This ratio of large HDL to total HDL particles varies widely and is measured only by more sophisticated lipoprotein assays using either electrophoresis (the original method developed in the 1970s) or newer NMR spectroscopy methods (See also nuclear magnetic resonance and spectroscopy), developed in the 1990s.
Subfractions
Five subfractions of HDL have been identified. From largest (and most effective in cholesterol removal) to smallest (and least effective), the types are 2a, 2b, 3a, 3b, and 3c.
Epidemiology
Men tend to have noticeably lower HDL concentrations, with smaller size and lower cholesterol content, than women. Men also have a greater incidence of atherosclerotic heart disease. Recent studies confirm the fact that HDL has a buffering role in balancing the effects of the hypercoagulable state in type 2 diabetics and decreases the high risk of cardiovascular complications in these patients. Also, the results obtained in this study revealed that there was a significant negative correlation between HDL and activated partial thromboplastin time (APTT).
Epidemiological studies have shown that high concentrations of HDL (over 60 mg/dL) have protective value against cardiovascular diseases such as ischemic stroke and myocardial infarction. Low concentrations of HDL (below 40 mg/dL for men, below 50 mg/dL for women) increase the risk for atherosclerotic diseases.
Data from the landmark Framingham Heart Study showed that, for a given level of LDL, the risk of heart disease increases 10-fold as the HDL varies from high to low. On the converse, however, for a fixed level of HDL, the risk increases 3-fold as LDL varies from low to high.
Even people with very low LDL levels under statins treatment are exposed to increased risk if their HDL levels are not high enough.
Estimating HDL via associated cholesterol
Clinical laboratories formerly measured HDL cholesterol by separating other lipoprotein fractions using either ultracentrifugation or chemical precipitation with divalent ions such as Mg2+, then coupling the products of a cholesterol oxidase reaction to an indicator reaction. The reference method still uses a combination of these techniques. Most laboratories now use automated homogeneous analytical methods in which lipoproteins containing apo B are blocked using antibodies to apo B, then a colorimetric enzyme reaction measures cholesterol in the non-blocked HDL particles. HPLC can also be used. Subfractions (HDL-2C, HDL-3C) can be measured, but clinical significance of these subfractions has not been determined. The measurement of apo-A reactive capacity can be used to measure HDL cholesterol but is thought to be less accurate.
Recommended ranges
The American Heart Association, NIH and NCEP provide a set of guidelines for fasting HDL levels and risk for heart disease.
| Level mg/dL | Level mmol/L | Interpretation |
|---|---|---|
| <40/50 men/women | <1.03 | Low HDL cholesterol, heightened risk considered correlated for heart disease |
| 40–59 | 1.03–1.55 | Medium HDL level |
| >59 | >1.55 | High HDL level, optimal condition considered correlated against heart disease |
High LDL with low HDL level is an additional risk factor for cardiovascular disease.
Measuring HDL concentration and sizes
As technology has reduced costs and clinical trials have continued to demonstrate the importance of HDL, methods for directly measuring HDL concentrations and size (which indicates function) at lower costs have become more widely available and increasingly regarded as important for assessing individual risk for progressive arterial disease and treatment methods.
Electrophoresis measurements
Since the HDL particles have a net negative charge and vary by density & size, ultracentrifugation combined with electrophoresis have been utilized since before 1950 to enumerate the concentration of HDL particles and sort them by size with a specific volume of blood plasma. Larger HDL particles are carrying more cholesterol.
NMR measurements
Concentration and sizes of lipoprotein particles can be estimated using nuclear magnetic resonance fingerprinting.
Optimal total and large HDL concentrations
The HDL particle concentrations are typically categorized by event rate percentiles based on the people participating and being tracked in the MESA trial, a medical research study sponsored by the United States National Heart, Lung, and Blood Institute.
| MESA Percentile | Total HDL particles μmol/L | Interpretation |
|---|---|---|
| >75% | >34.9 | Those with highest (Optimal) total HDL particle concentrations & lowest rates of cardiovascular disease events |
| 50–75% | 30.5–34.5 | Those with moderately high total HDL particle concentrations & moderate rates of cardiovascular disease events |
| 25–50% | 26.7–30.5 | Those with lower total HDL particle concentrations & Borderline-High rates of cardiovascular disease |
| 0–25% | <26.7 | Those with lowest total HDL particle concentrations & Highest rates of cardiovascular disease events |
| MESA Percentile | Large HDL particles μmol/L | Interpretation |
|---|---|---|
| >75% | >7.3 | Those with highest (Optimal) Large HDL particle concentrations & lowest rates of cardiovascular disease events |
| 50–75% | 4.8–7.3 | Those with moderately high Large HDL particle concentrations & moderate rates of cardiovascular disease events |
| 25–50% | 3.1–4.8 | Those with lower Large HDL particle concentrations & Borderline-High rates of cardiovascular disease |
| 0–25% | <3.1 | Those with lowest Large HDL particle concentrations & Highest rates of cardiovascular disease events |
The lowest incidence of atherosclerotic events over time occurs within those with both the highest concentrations of total HDL particles (the top quarter, >75%) and the highest concentrations of large HDL particles. Multiple additional measures, including LDL particle concentrations, small LDL particle concentrations, VLDL concentrations, estimations of insulin resistance and standard cholesterol lipid measurements (for comparison of the plasma data with the estimation methods discussed above) are routinely provided in clinical testing.
Increasing HDL levels
While higher HDL levels are correlated with lower risk of cardiovascular diseases, no medication used to increase HDL has been proven to improve health. As of 2017, numerous lifestyle changes and drugs to increase HDL levels were under study.
HDL lipoprotein particles that bear apolipoprotein C3 are associated with increased, rather than decreased, risk for coronary heart disease.
Diet and exercise
Certain changes in diet and exercise may have a positive impact on raising HDL levels:
- Decreased intake of simple carbohydrates.
- Aerobic exercise
- Weight loss
- Avocado consumption
- Magnesium supplements raise HDL-C.
- Addition of soluble fiber to diet
- Consumption of omega-3 fatty acids such as fish oil or flax oil
- Increased intake of unsaturated fats
- Removal of trans fatty acids from the diet
Most saturated fats increase HDL cholesterol to varying degrees but also raise total and LDL cholesterol.
Recreational drugs
HDL levels can be increased by smoking cessation, or mild to moderate alcohol intake.
Cannabis in unadjusted analyses, past and current cannabis use was not associated with higher HDL-C levels. A study performed in 4635 patients demonstrated no effect on the HDL-C levels (P=0.78) [the mean (standard error) HDL-C values in control subjects (never used), past users and current users were 53.4 (0.4), 53.9 (0.6) and 53.9 (0.7) mg/dL, respectively].
Exogenous anabolic androgenic steroids, particularly 17α-alkylated anabolic steroids and others administered orally, can reduce HDL-C by 50 percent or more. Other androgen receptor agonists such as selective androgen receptor modulators can also lower HDL. As there is some evidence that the HDL reduction is caused by increased reverse cholesterol transport, it is unknown if AR agonists' HDL-lowering effect is pro- or anti-atherogenic.
Pharmaceutical drugs and niacin
Pharmacological therapy to increase the level of HDL cholesterol includes use of fibrates and niacin. Fibrates have not been proven to have an effect on overall deaths from all causes, despite their effects on lipids.
Niacin (nicotinic acid, a form of vitamin B3) increases HDL by selectively inhibiting hepatic diacylglycerol acyltransferase 2, reducing triglyceride synthesis and VLDL secretion through a receptor HM74 otherwise known as niacin receptor 2 and HM74A / GPR109A, niacin receptor 1.
Pharmacologic (1- to 3-gram/day) niacin doses increase HDL levels by 10–30%, making it the most powerful agent to increase HDL-cholesterol. A randomized clinical trial demonstrated that treatment with niacin can significantly reduce atherosclerosis progression and cardiovascular events. Niacin products sold as "no-flush", i.e. not having side-effects such as "niacin flush", do not, however, contain free nicotinic acid and are therefore ineffective at raising HDL, while products sold as "sustained-release" may contain free nicotinic acid, but "some brands are hepatotoxic"; therefore the recommended form of niacin for raising HDL is the cheapest, immediate-release preparation. Both fibrates and niacin increase artery toxic homocysteine, an effect that can be counteracted by also consuming a multivitamin with relatively high amounts of the B-vitamins, but multiple European trials of the most popular B-vitamin cocktails, trial showing 30% average reduction in homocysteine, while not showing problems have also not shown any benefit in reducing cardiovascular event rates. A 2011 extended-release niacin (Niaspan) study was halted early because patients adding niacin to their statin treatment showed no increase in heart health, but did experience an increase in the risk of stroke.
In contrast, while the use of statins is effective against high levels of LDL cholesterol, most have little or no effect in raising HDL cholesterol.
Lovaza has been shown to increase HDL-C. However, the best evidence to date suggests it has no benefit for primary or secondary prevention of cardiovascular disease.
The PPAR modulator GW501516 has shown a positive effect on HDL-C and an antiatherogenic where LDL is an issue. However, research on the drug has been discontinued after it was discovered to cause rapid cancer development in several organs in rats.