ヒドロキソコバラミン
Hydroxocobalamin/ja
ヒドロキソコバラミン(hydroxocobalamin)は、ビタミンB12aやヒドロキシコバラミンとしても知られるビタミンの一種で、食品中に含まれ、栄養補助食品として用いられる。サプリメントとしては、悪性貧血を含むビタミンB12欠乏症の治療に用いられる。その他の用途としては、シアン中毒、レーバー視神経萎縮症、中毒性弱視の治療がある。筋肉内または静脈内に注射する。
 | |
| Clinical data | |
|---|---|
| Other names | vitamin B12, vitamin B12a, hydroxycobalamin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605007 |
| Routes of administration | Intramuscular, intravenous/ja |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Very high (90%) |
| Metabolism | 主に肝臓で、コバラミンは回腸で吸収され、肝臓に貯蔵される。 |
| Elimination half-life | ~6 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C62H89CoN13O15P |
| Molar mass | 1346.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
副作用は一般的にほとんどない。下痢、気分の悪さ、ほてり、かゆみ、低血中カリウム、アレルギー反応、高血圧などである。通常量は妊娠中に安全と考えられている。この薬物による過量投与や毒性は報告されていない。ヒドロキソコバラミン(hydroxocobalamin)は、ビタミンB12の天然型であり、化合物のコバラミンファミリーのメンバーである。ヒドロキソコバラミン、またはビタミンB12の別の形態は、体内でDNAを作るために必要である。
ヒドロキソコバラミンは1949年に初めて単離された。WHO必須医薬品リストに掲載されている。ヒドロキソコバラミンはジェネリック医薬品として販売されている。
医療用途
ビタミンB12欠乏症
ビタミンB12欠乏症の治療の標準治療は、ヒドロキソコバラミン(OHCbl)の筋肉内(IM)または静脈内(IV)注射である。というのも、大半の症例は経腸ルート(腸)での吸収不良によるものだからである。本態性コバラミン代謝性疾患の小児患者、青酸中毒によるタバコ弱視のビタミンB12欠乏患者、視神経障害のある悪性貧血患者などに用いられる。
新たにビタミンB12欠乏症と診断された患者(通常、血清中濃度が200 pg/ml未満と定義される)では、消耗したコバラミン貯蔵量を補充するために、1日1,000 μg(1 mg)までのヒドロキソコバラミン注射を毎日IM注射する。神経症状がある場合は、毎日の治療後、6ヵ月間は週1回または隔週1回の注射を行い、その後月1回のIM注射を開始する。臨床的な改善が確認されれば、B12の維持補充が一般的に生涯必要となる。
シアン中毒
ヒドロキソコバラミン(Hydroxocobalamin)は、シアン中毒の患者に対する第一選択薬である。ヒドロキソコバラ ミンはシアンをはるかに毒性の低いシアノコバラミンに変換する。シアノコバラミンは腎で排出される。ヒドロキソコバラミンが第一選択薬となったのは、その有害リスクプロファイルの低さ、作用発現の速さ、病院前での使いやすさによるものである。
Injectable hydroxocobalamin
Injection of hydroxocobalamin is used to rectify the following causes of vitamin B12 deficiency (list taken from the drug prescription label published by the U.S. Food and Drug Administration)
- Dietary deficiency of vitamin B12 occurring in strict vegetarians and in their breastfed infants (isolated vitamin B12 deficiency is very rare)
- Malabsorption of vitamin B12 resulting from damage to the stomach, where intrinsic factor is secreted, or damage to the ileum, where intrinsic factor facilitates vitamin B12 absorption. These conditions include tropical sprue and nontropical sprue (celiac disease).
- Inadequate secretion of intrinsic factor, resulting from lesions that destroy the gastric mucosa (which can be caused by ingestion of corrosives, extensive tumors, and conditions associated with gastric atrophy, such as multiple sclerosis, certain endocrine disorders, iron deficiency, and subtotal gastrectomy)
- Structural lesions leading to vitamin B12 deficiency, including regional ileitis, ileal reactions, and malignancies
- Competition for vitamin B12 by intestinal parasites or bacteria. The tapeworm from undercooked fish (Diphyllobothrium latum) absorbs huge quantities of vitamin B12, and infested patients often have associated gastric atrophy. The blind loop syndrome may produce deficiency of vitamin B12 or folate.
- Inadequate use of vitamin B12, which may occur if antimetabolites for the vitamin are employed in the treatment of neoplasia
Pernicious anemia is the most common cause of vitamin B12 deficiency. While it technically refers to anemia caused specifically by autoimmune deficiency of intrinsic factor, it is commonly used to refer to B12-deficient anemia as a whole, regardless of cause.
Side effects
The literature data on the acute toxicity profile of hydroxocobalamin show it is generally regarded as safe with local and systemic exposure. The ability of hydroxocobalamin to rapidly scavenge and detoxify cyanide by chelation has resulted in several acute animal and human studies using systemic hydroxocobalamin doses at suprapharmacological doses as high as 140 mg/kg to support its use as an intravenous (IV) treatment for cyanide exposure. The US FDA at the end of 2006 approved the use hydroxocobalamin as an injection for the treatment of cyanide poisoning.
The drug causes a reddish discoloration of the urine (chromaturia), which can look like blood in the urine.
Properties
Hydroxocobalamin acetate occurs as odorless, dark-red orthorhombic crystals. The injection formulations appear as clear, dark-red solutions. It has a distribution coefficient of 1.133×10−5 and a pKa of 7.65.
Mechanism of action
Vitamin B12 refers to a group of compounds called cobalamins that are available in the human body in a variety of mostly interconvertible forms. Together with folate, cobalamins are essential cofactors required for DNA synthesis in cells where chromosomal replication and division are occurring—most notably the bone marrow and myeloid cells. As a cofactor, cobalamins are essential for two cellular reactions:
- the mitochondrial methylmalonyl-CoA mutase conversion of methylmalonic acid (MMA) to succinate, which links lipid and carbohydrate metabolism, and
- the activation of methionine synthase, which is the rate-limiting step in the synthesis of methionine from homocysteine and 5-methyltetrahydrofolate.
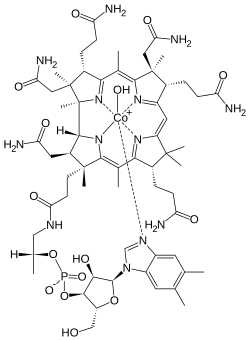
Cobalamins are characterized by a porphyrin-like corrin nucleus that contains a single cobalt atom bound to a benzimidazolyl nucleotide and a variable residue (R) group. The variable R group gives rise to the four most commonly known cobalamins: CNCbl, methylcobalamin, 5-deoxyadenosylcobalamin, and OHCbl. In the serum, hydroxocobalamin and cyanocobalamin are believed to function as storage or transport forms of the molecule, whereas methylcobalamin and 5-deoxyadenosylcobalamin are the active forms of the coenzyme required for cell growth and replication. Cyanocobalamin is usually converted to hydroxocobalamin in the serum, whereas hydroxocobalamin is converted to either methylcobalamin or 5-deoxyadenosyl cobalamin. Cobalamins circulate bound to serum proteins called transcobalamins (TC) and haptocorrins. Hydroxocobalamin has a higher affinity to the TC II transport protein than cyanocobalamin, or 5-deoxyadenosylcobalamin. From a biochemical point of view, two essential enzymatic reactions require vitamin B12 (cobalamin).
Intracellular vitamin B12 is maintained in two active coenzymes, methylcobalamin and 5-deoxyadenosylcobalamin. In the face of vitamin B12 deficiency, conversion of methylmalonyl-CoA to succinyl-CoA cannot take place, which results in accumulation of methylmalonyl-CoA and aberrant fatty acid synthesis. In the other enzymatic reaction, methylcobalamin supports the methionine synthase reaction, which is essential for normal metabolism of folate. The folate-cobalamin interaction is pivotal for normal synthesis of purines and pyrimidines and the transfer of the methyl group to cobalamin is essential for the adequate supply of tetrahydrofolate, the substrate for metabolic steps that require folate. In a state of vitamin B12 deficiency, the cell responds by redirecting folate metabolic pathways to supply increasing amounts of methyltetrahydrofolate. The resulting elevated concentrations of homocysteine and MMA are often found in patients with low serum vitamin B12 and can usually be lowered with successful vitamin B12 replacement therapy. However, elevated MMA and homocysteine concentrations may persist in patients with cobalamin concentrations between 200 and 350 pg/mL. Supplementation with vitamin B12 during conditions of deficiency restores the intracellular level of cobalamin and maintains a sufficient level of the two active coenzymes: methylcobalamin and deoxyadenosylcobalamin.