リボフラビン
Riboflavin/ja
 | |
 化学構造 | |
| Clinical data | |
|---|---|
| Trade names | Many |
| Other names | lactochrome, lactoflavin, vitamin G |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | 口から, intramuscular/ja, intravenous/ja |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 66 to 84 minutes |
| Excretion | 尿 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H20N4O6 |
| Molar mass | 376.369 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
リボフラビン(Riboflavin)は、ビタミンB2としても知られ、食品に含まれるビタミンの一種であり、栄養補助食品として販売されている。2つの主要な補酵素、フラビンモノヌクレオチドとフラビンアデニンジヌクレオチドの形成に必須である。これらの補酵素は、エネルギー代謝、細胞呼吸、抗体産生、および正常な成長と発達に関与している。補酵素はまた、ナイアシン、ビタミンB6、葉酸の代謝にも必要である。リボフラビンは角膜菲薄化を治療するために処方薬されており、経口摂取することで成人の片頭痛の発生率を低下させる可能性がある。
リボフラビン欠乏症はまれで、通常は他のビタミンや栄養素の欠乏を伴う。経口サプリメントまたは注射によって予防または治療することができる。水溶性ビタミンであるリボフラビンは、栄養所要量を超えて摂取しても貯蔵されることはなく、吸収されないか、吸収されてすぐに尿中に排出され、尿が鮮やかな黄色を帯びる。リボフラビンの天然供給源としては、肉、魚、家禽、卵、乳製品、緑黄色野菜、キノコ類、アーモンドなどがある。国によっては、穀類への添加を義務付けているところもある。
リボフラビンは1920年に発見され、1933年に単離され、1935年に初めて合成された。精製された固体の状態では、水溶性の黄橙色の結晶性の粉末である。ビタミンとしての機能に加えて、食品着色料として使用される。生合成は細菌、菌類、植物で行われるが、動物では行われない。リボフラビンの工業的合成は当初化学的プロセスで達成されたが、現在の商業的製造は菌類の菌株や遺伝子組み換え細菌を用いた発酵法に依存している。
定義
ビタミンB2としても知られるリボフラビンは、水溶性のビタミンであり、ビタミンB群の一つである。葉酸やビタミンB6がビタマーとして知られるいくつかの化学的に関連した形で存在するのとは異なり、リボフラビンは1つの化学化合物のみである。それは補酵素フラビンモノヌクレオチド(FMN、リボフラビン-5'-リン酸としても知られる)とフラビンアデニンジヌクレオチド(FAD)の合成における出発化合物である。FADはフラビンの中でもより豊富に存在し、全種類のゲノム(フラボプロテオーム)においてフラビン依存性タンパク質がコードする遺伝子数の75%と結合していると報告されており、ヒトがコードするフラボタンパク質の84%の補酵素として機能している。
リボフラビンは精製された固体の状態では黄橙色の結晶線粉末で、わずかに臭いと苦味がある。水や塩化ナトリウム水溶液などの極性溶媒に溶け、アルコールにもわずかに溶ける。クロロホルム、ベンゼン、アセトンなどの非極性または弱極性有機溶媒には溶けない。リボフラビンは、溶液中または粉末として乾燥保存している間は、光にさらされなければ熱に安定である。加熱して分解すると、一酸化窒素を含む有毒ガスを放出する。
機能
リボフラビンは、2つの主要な補酵素であるFMNとFADの形成に必須である。これらの補酵素はエネルギー代謝、細胞呼吸、抗体産生、成長および発達に関与する。リボフラビンは炭水化物、タンパク質、脂肪の代謝に必須である。FADはトリプトファンからナイアシン(ビタミンB3)への変換に寄与し、ビタミンB6から補酵素ピリドキサール5'-リン酸への変換にはFMNが必要である。リボフラビンは、ホモシステインの正常な循環レベルの維持に関与している。リボフラビン欠乏症では、ホモシステインレベルが上昇し、心血管疾患のリスクが上昇する。
酸化還元反応
酸化還元反応は電子伝達を伴うプロセスである。フラビン補酵素は、酸化型、半還元型、完全還元型の間で変換されるフラビンの能力を利用した1電子または2電子の酸化還元反応を担う、ヒトのおよそ70〜80種類のフラビン酵素(始原菌、細菌、真菌のゲノムにコードされているものを含む、すべての生物全体で数百種類以上)の機能を支えている。FADはまた、内因性の抗酸化物質グルタチオンの形成に不可欠な酵素であるグルタチオン還元酵素の活性にも必要である。
微量栄養素の代謝
リボフラビン、FMN、FADはナイアシン、ビタミンB6、葉酸の代謝に関与する。トリプトファンからのナイアシン含有補酵素であるNADとNADPの合成には、FAD依存性酵素であるキヌレニン3モノオキシゲナーゼが関与している。リボフラビンの欠乏はNADとNADPの産生を減少させ、ナイアシン欠乏を促進する。ビタミンB6からその補酵素であるピリドキサール5'-リン酸合成酵素への変換には、FMNを必要とするピリドキシン5'-リン酸オキシダーゼという酵素が関与する。葉酸代謝に関与する酵素である5,10-メチレンテトラヒドロ葉酸還元酵素は、FMNを必要とする。ホモシステインからアミノ酸であるメチオニンを形成するためにFADを必要とする。
リボフラビンの欠乏は、ヘモグロビンや赤血球の生成に不可欠な栄養ミネラルである鉄の代謝を損なうようである。リボフラビンと鉄の両方が欠乏している人のリボフラビン欠乏を緩和することは、鉄欠乏性貧血を治療するための鉄サプリメントの効果を改善する。
合成
生合成
生合成は細菌、菌類、植物で行われるが、動物では行われない。リボフラビンの生合成前駆体はリブロース5-リン酸とグアノシン3リン酸である。前者はL-3,4-ジヒドロキシ-2-ブタノン-4-リン酸に変換され、後者は一連の反応で5-アミノ-6-(D-リビチルアミノ)ウラシルに変換される。これら2つの化合物は、酵素ルマジン合成酵素の反応EC 2.5.1.78によって触媒される、経路の最後の段階の基質となる。
生合成の最終段階では、2分子の6,7-ジメチル-8-リビチルルマジンが酵素リボフラビン合成酵素によって脱離反応で結合される。これによりリボフラビン1分子と5-アミノ-6-(D-リビチルアミノ)ウラシル1分子が生成される。後者は前の反応にリサイクルされる。
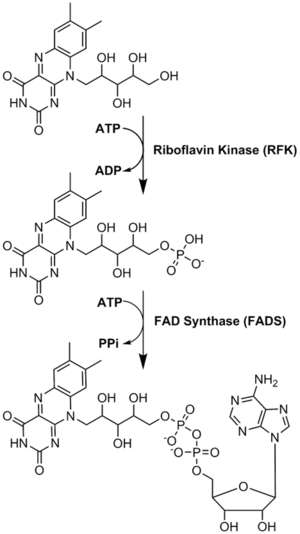
リボフラビンから補酵素への変換が行われる。FMNとFADは、酵素リボフラビンキナーゼとFAD合成酵素が順次作用することによって行われる。
工業合成

リボフラビンの工業的生産には、Ashbya gossypii、Candida famata、Candida flaveriなどの糸状菌や、細菌のCorynebacterium ammoniagenes、枯草菌などを含む様々な微生物が用いられる。リボフラビンの生産量を増やし、抗生物質(アンピシリン)耐性マーカーを導入するために遺伝子組み換えされた枯草菌は、飼料や食品強化のためのリボフラビン生産に商業規模で使用されている。2012年までに、このような発酵プロセスによって年間4,000トン以上が生産された。
高濃度の炭化水素や芳香族化合物が存在すると、一部の細菌はリボフラビンを過剰に産生する。そのような菌の1つがマイクロコッカス・ルテウス(アメリカン・タイプ・カルチャー・コレクション菌株番号ATCC 49442)で、ピリジン上で増殖するとリボフラビンの産生により黄色に発色するが、コハク酸などの他の基質上で増殖すると発色しない。
実験室での合成
リボフラビンの最初の全合成はRichard Kuhnのグループによって行われた。D-リボースを用いた還元的アミノ化によって生成した置換アニリンを、最終段階でアロキサンと縮合させた:
用途
角膜菲薄化の治療
円錐角膜は角膜外反の最も一般的な形態である。角膜が徐々に薄くなる病気であり、この症状は、角膜の硬度を高める角膜コラーゲンクロスリンキングによって治療される。クロスリンキングは、局所リボフラビン溶液を角膜に塗布し、紫外線 A光を照射することで達成される。
片頭痛予防
2012年のガイドラインで米国神経学会は、高用量リボフラビン(400 mg)は「おそらく有効であり、片頭痛予防のために考慮すべきである」と述べており、これは英国国立片頭痛センターも推奨している。2017年のレビューでは、リボフラビンを1日400 mg、少なくとも3ヵ月間毎日摂取することで、成人の片頭痛の頻度が減少する可能性があると報告されている。小児および青年における片頭痛予防または治療のための高用量リボフラビンに関する研究は結論が出ていないため、サプリメントは推奨されていない。
食品着色料
リボフラビンは食品着色料(黄橙色の結晶性粉末)として使用され、ヨーロッパでは食品添加物として使用するためにE101というE番号で指定されている。
Dietary recommendations
The National Academy of Medicine updated the Estimated Average Requirements (EARs) and Recommended Dietary Allowances (RDAs) for riboflavin in 1998. The EARs[update] for riboflavin for women and men aged 14 and over are 0.9 mg/day and 1.1 mg/day, respectively; the RDAs are 1.1 and 1.3 mg/day, respectively. RDAs are higher than EARs to provide adequate intake levels for individuals with higher than average requirements. The RDA during pregnancy is 1.4 mg/day and the RDA for lactating females is 1.6 mg/day. For infants up to the age of 12 months, the Adequate Intake (AI) is 0.3–0.4 mg/day and for children aged 1–13 years the RDA increases with age from 0.5 to 0.9 mg/day. As for safety, the IOM sets tolerable upper intake levels (ULs) for vitamins and minerals when evidence is sufficient. In the case of riboflavin there is no UL, as there is no human data for adverse effects from high doses. Collectively the EARs, RDAs, AIs and ULs are referred to as Dietary Reference Intakes (DRIs).
The European Food Safety Authority (EFSA) refers to the collective set of information as Dietary Reference Values, with Population Reference Intake (PRI) instead of RDA, and Average Requirement instead of EAR. AI and UL are defined the same as in United States. For women and men aged 15 and older the PRI is set at 1.6 mg/day. The PRI during pregnancy is 1.9 mg/day and the PRI for lactating females is 2.0 mg/day. For children aged 1–14 years the PRIs increase with age from 0.6 to 1.4 mg/day. These PRIs are higher than the U.S. RDAs. The EFSA also considered the maximum safe intake and like the U.S. National Academy of Medicine, decided that there was not sufficient information to set an UL.
| Recommended Dietary Allowances United States | |
| Age group (years) | RDA for riboflavin (mg/d) |
|---|---|
| 0–6 months | 0.3* |
| 6–12 months | 0.4* |
| 1–3 | 0.5 |
| 4–8 | 0.6 |
| 9–13 | 0.9 |
| Females 14–18 | 1.0 |
| Males 14–18 | 1.3 |
| Females 19+ | 1.1 |
| Males 19+ | 1.3 |
| Pregnant females | 1.4 |
| Lactating females | 1.6 |
| * Adequate intake for infants, no RDA/RDI yet established | |
| Population Reference Intakes European Union | |
| Age group (years) | PRI for riboflavin (mg/d) |
| 7–11 months | 0.4 |
| 1–3 | 0.6 |
| 4–6 | 0.7 |
| 7–10 | 1.0 |
| 11–14 | 1.4 |
| 15–adult | 1.6 |
| Pregnant females | 1.9 |
| Lactating females | 2.0 |
Safety
In humans, there is no evidence for riboflavin toxicity produced by excessive intakes and absorption becomes less efficient as dosage increases. Any excess riboflavin is excreted via the kidneys into urine, resulting in a bright yellow color known as flavinuria. During a clinical trial on the effectiveness of riboflavin for treating the frequency and severity of migraines, subjects were given up to 400 mg of riboflavin orally per day for periods of 3–12 months. Abdominal pains and diarrhea were among the side effects reported.
Labeling
For U.S. food and dietary supplement labeling purposes the amount in a serving is expressed as a percent of Daily Value (%DV). For riboflavin labeling purposes 100% of the Daily Value was 1.7 mg, but as of May 27, 2016, it was revised to 1.3 mg to bring it into agreement with the RDA. A table of the old and new adult daily values is provided at Reference Daily Intake.
Sources
The United States Department of Agriculture, Agricultural Research Service maintains a food composition database from which riboflavin content in hundreds of foods can be searched.
|
|
|
The milling of wheat results in an 85% loss of riboflavin, so white flour is enriched in some countries. Riboflavin is also added to baby foods, breakfast cereals, pastas and vitamin-enriched meal replacement products. It is difficult to incorporate riboflavin into liquid products because it has poor solubility in water, hence the requirement for riboflavin-5'-phosphate (FMN, also called E101 when used as colorant), a more soluble form of riboflavin. The enrichment of bread and ready-to-eat breakfast cereals contributes significantly to the dietary supply of the vitamin. Free riboflavin is naturally present in animal-sourced foods along with protein-bound FMN and FAD. Cows' milk contains mainly free riboflavin, but both FMN and FAD are present at low concentrations.
Fortification
Some countries require or recommend fortification of grain foods. As of 2021, 56 countries, mostly in North and South America and southeast Africa, require food fortification of wheat flour or maize (corn) flour with riboflavin or riboflavin-5'-phosphate sodium. The amounts stipulated range from 1.3 to 5.75 mg/kg. An additional 16 countries have a voluntary fortification program. For example, the Indian government recommends 4.0 mg/kg for "maida" (white) and "atta" (whole wheat) flour.
Absorption, metabolism, excretion
More than 90% of riboflavin in the diet is in the form of protein-bound FMN and FAD. Exposure to gastric acid in the stomach releases the coenzymes, which are subsequently enzymatically hydrolyzed in the proximal small intestine to release free riboflavin.
Absorption occurs via a rapid active transport system, with some additional passive diffusion occurring at high concentrations. Bile salts facilitate uptake, so absorption is improved when the vitamin is consumed with a meal. One small clinical trial in adults reported that the maximum amount of riboflavin that can be absorbed from a single dose is 27 mg.> The majority of newly absorbed riboflavin is taken up by the liver on the first pass, indicating that postprandial appearance of riboflavin in blood plasma may underestimate absorption. Three riboflavin transporter proteins have been identified: RFVT1 is present in the small intestine and also in the placenta; RFVT2 is highly expressed in brain and salivary glands; and RFVT3 is most highly expressed in the small intestine, testes, and prostate. Infants with mutations in the genes encoding these transport proteins can be treated with riboflavin administered orally.
Riboflavin is reversibly converted to FMN and then FAD. From riboflavin to FMN is the function of zinc-requiring riboflavin kinase; the reverse is accomplished by a phosphatase. From FMN to FAD is the function of magnesium-requiring FAD synthase; the reverse is accomplished by a pyrophosphatase. FAD appears to be an inhibitory end-product that down-regulates its own formation.
When excess riboflavin is absorbed by the small intestine, it is quickly removed from the blood and excreted in urine. Urine color is used as a hydration status biomarker and, under normal conditions, correlates with urine specific gravity and urine osmolality. However, riboflavin supplementation in large excess of requirements causes urine to appear more yellow than normal. With normal dietary intake, about two-thirds of urinary output is riboflavin, the remainder having been partially metabolized to hydroxymethylriboflavin from oxidation within cells, and as other metabolites. When consumption exceeds the ability to absorb, riboflavin passes into the large intestine, where it is catabolized by bacteria to various metabolites that can be detected in feces. There is speculation that unabsorbed riboflavin could affect the large intestine microbiome.
Deficiency
Prevalence
Riboflavin deficiency is uncommon in the United States and in other countries with wheat flour or corn meal fortification programs. From data collected in biannual surveys of the U.S. population, for ages 20 and over, 22% of females and 19% of men reported consuming a supplement that contained riboflavin, typically a vitamin-mineral multi-supplement. For the non-supplement users, the dietary intake of adult women averaged 1.74 mg/day and men 2.44 mg/day. These amounts exceed the RDAs for riboflavin of 1.1 and 1.3 mg/day respectively. For all age groups, on average, consumption from food exceeded the RDAs. A 2001-02 U.S. survey reported that less than 3% of the population consumed less than the Estimated Average Requirement of riboflavin.
Signs and symptoms
Riboflavin deficiency (also called ariboflavinosis) results in stomatitis, symptoms of which include chapped and fissured lips, inflammation of the corners of the mouth (angular stomatitis), sore throat, painful red tongue, and hair loss. The eyes can become itchy, watery, bloodshot, and sensitive to light. Riboflavin deficiency is associated with anemia. Prolonged riboflavin insufficiency may cause degeneration of the liver and nervous system. Riboflavin deficiency may increase the risk of preeclampsia in pregnant women. Deficiency of riboflavin during pregnancy can result in fetal birth defects, including heart and limb deformities.
Risk factors
People at risk of having low riboflavin levels include alcoholics, vegetarian athletes, and practitioners of veganism. Pregnant or lactating women and their infants may also be at risk, if the mother avoids meat and dairy products. Anorexia and lactose intolerance increase the risk of riboflavin deficiency. People with physically demanding lives, such as athletes and laborers, may require higher riboflavin intake. The conversion of riboflavin into FAD and FMN is impaired in people with hypothyroidism, adrenal insufficiency, and riboflavin transporter deficiency.
Causes
Riboflavin deficiency is usually found together with other nutrient deficiencies, particularly of other water-soluble vitamins. A deficiency of riboflavin can be primary (i.e. caused by poor vitamin sources in the regular diet) or secondary, which may be a result of conditions that affect absorption in the intestine. Secondary deficiencies are typically caused by the body not being able to use the vitamin, or by an increased rate of excretion of the vitamin. Diet patterns that increase risk of deficiency include veganism and low-dairy vegetarianism. Diseases such as cancer, heart disease and diabetes may cause or exacerbate riboflavin deficiency.
There are rare genetic defects that compromise riboflavin absorption, transport, metabolism or use by flavoproteins. One of these is riboflavin transporter deficiency, previously known as Brown–Vialetto–Van Laere syndrome. Variants of the genes SLC52A2 and SLC52A3 which code for transporter proteins RDVT2 and RDVT3, respectively, are defective. Infants and young children present with muscle weakness, cranial nerve deficits including hearing loss, sensory symptoms including sensory ataxia, feeding difficulties, and respiratory distress caused by a sensorimotor axonal neuropathy and cranial nerve pathology. When untreated, infants with riboflavin transporter deficiency have labored breathing and are at risk of dying in the first decade of life. Treatment with oral supplementation of high amounts of riboflavin is lifesaving.
Other inborn errors of metabolism include riboflavin-responsive multiple acyl-CoA dehydrogenase deficiency, also known as a subset of glutaric acidemia type 2, and the C677T variant of the methylenetetrahydrofolate reductase enzyme, which in adults has been associated with risk of high blood pressure.
Diagnosis and assessment
The assessment of riboflavin status is essential for confirming cases with non-specific symptoms whenever deficiency is suspected. Total riboflavin excretion in healthy adults with normal riboflavin intake is about 120 micrograms per day, while excretion of less than 40 micrograms per day indicates deficiency. Riboflavin excretion rates decrease as a person ages, but increase during periods of chronic stress and the use of some prescription drugs.
Indicators used in humans are erythrocyte glutathione reductase (EGR), erythrocyte flavin concentration and urinary excretion. The erythrocyte glutathione reductase activity coefficient (EGRAC) provides a measure of tissue saturation and long-term riboflavin status. Results are expressed as an activity coefficient ratio, determined by enzyme activity with and without the addition of FAD to the culture medium. An EGRAC of 1.0 to 1.2 indicates that adequate amounts of riboflavin are present; 1.2 to 1.4 is considered low, greater than 1.4 indicates deficient. For the less sensitive "erythrocyte flavin method", values greater than 400 nmol/L are considered adequate and values below 270 nmol/L are considered deficient. Urinary excretion is expressed as nmol of riboflavin per gram of creatinine. Low is defined as in the range of 50 to 72 nmol/g. Deficient is below 50 nmol/g. Urinary excretion load tests have been used to determine dietary requirements. For adult men, as oral doses were increased from 0.5 mg to 1.1 mg, there was a modest linear increase in urinary riboflavin, reaching 100 micrograms for a subsequent 24-hour urine collection.Beyond a load dose of 1.1 mg, urinary excretion increased rapidly, so that with a dose of 2.5 mg, urinary output was 800 micrograms for a 24-hour urine collection.
History
The name "riboflavin" comes from "ribose" (the sugar whose reduced form, ribitol, forms part of its structure) and "flavin", the ring-moiety that imparts the yellow color to the oxidized molecule (from Latin flavus, "yellow"). The reduced form, which occurs in metabolism along with the oxidized form, appears as orange-yellow needles or crystals. The earliest reported identification, predating any concept of vitamins as essential nutrients, was by Alexander Wynter Blyth. In 1879, Blyth isolated a water-soluble component of cows' milk whey, which he named "lactochrome", that fluoresced yellow-green when exposed to light.
In the early 1900s, several research laboratories were investigating constituents of foods, essential to maintain growth in rats. These constituents were initially divided into fat-soluble "vitamine" A and water-soluble "vitamine" B. (The "e" was dropped in 1920.) Vitamin B was further thought to have two components, a heat-labile substance called B1 and a heat-stable substance called B2. Vitamin B2 was tentatively identified to be the factor necessary for preventing pellagra, but that was later confirmed to be due to niacin (vitamin B3) deficiency. The confusion was due to the fact that riboflavin (B2) deficiency causes stomatitis symptoms similar to those seen in pellagra, but without the widespread peripheral skin lesions. For this reason, early in the history of identifying riboflavin deficiency in humans the condition was sometimes called "pellagra sine pellagra" (pellagra without pellagra).
In 1935, Paul Gyorgy, in collaboration with chemist Richard Kuhn and physician T. Wagner-Jauregg, reported that rats kept on a B2-free diet were unable to gain weight. Isolation of B2 from yeast revealed the presence of a bright yellow-green fluorescent product that restored normal growth when fed to rats. The growth restored was directly proportional to the intensity of the fluorescence. This observation enabled the researchers to develop a rapid chemical bioassay in 1933, and then isolate the factor from egg white, calling it ovoflavin. The same group then isolated the a similar preparation from whey and called it lactoflavin. In 1934, Kuhn's group identified the chemical structure of these flavins as identical, settled on "riboflavin" as a name, and were also able to synthesize the vitamin.
Circa 1937, riboflavin was also referred to as "Vitamin G". In 1938, Richard Kuhn was awarded the Nobel Prize in Chemistry for his work on vitamins, which had included B2 and B6. In 1939, it was confirmed that riboflavin is essential for human health through a clinical trial conducted by William H. Sebrell and Roy E. Butler. Women fed a diet low in riboflavin developed stomatitis and other signs of deficiency, which were reversed when treated with synthetic riboflavin. The symptoms returned when the supplements were stopped.