脂肪組織
Adipose tissue/ja
脂肪組織(体脂肪または単に脂肪とも呼ばれる)は、主に脂肪細胞から構成される緩い結合組織である。また、前脂肪細胞、線維芽細胞、血管内皮細胞や脂肪組織マクロファージなどの様々な免疫細胞の細胞の間質血管分画(SVF)も含んでいる。主な役割は脂質の形でエネルギーを貯蔵することであるが、クッションや保温も行っている。
| 脂肪組織 | |
|---|---|
 豚の腹の脂肪(白) | |
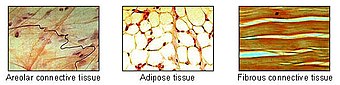 脂肪組織は結合組織の主な種類の一つである。 | |
| Pronunciation | /ˈædɪˌpoʊs/ ( |
| Anatomical terminology | |
以前はホルモンに不活性な臓器として扱われていたが、近年、脂肪組織はレプチン、エストロゲン、レジスチン、サイトカイン(特にTNFα)などのホルモンを産生するため、主要な内分泌臓器として認識されている。肥満では、脂肪組織はアディポカインとして知られる炎症性マーカーの慢性的な放出に関与しており、メタボリックシンドローム—(2型糖尿病、心血管系疾患、アテローム性動脈硬化症を含む一連の疾患の発症に関与している。
脂肪組織は前駆脂肪細胞に由来し、その形成は部分的に脂肪遺伝子によって制御されているようである。脂肪組織には、エネルギーを貯蔵する白色脂肪組織(WAT)と、体温を発生させる褐色脂肪組織(BAT)の2種類がある。脂肪組織—、特に褐色脂肪組織—は、1551年にスイスの博物学者コンラッド・ゲスナーによって初めて同定された。
解剖学的特徴
人間には脂肪組織がある:皮膚の下(皮下脂肪)、内部の臓器の周囲(内臓脂肪)、骨髄(黄色骨髄)、筋肉間(筋肉系)、乳房(乳腺組織)に存在する。脂肪組織は特定の場所に存在し、それらは脂肪デポと呼ばれている。脂肪組織内の細胞の中で最も高い割合を占める脂肪細胞以外に、間質血管分画(SVF)と総称される他のタイプの細胞も存在する。SVFには前脂肪細胞、線維芽細胞、脂肪組織マクロファージ、内皮細胞が含まれる。
脂肪組織は多くの小さな血管を含んでいる。皮膚を含む表皮系では、最深部である皮下層に蓄積し、熱や寒さからの断熱を提供する。 臓器の周囲では、保護パッドを提供している。しかし、その主な機能は脂質の備蓄であり、身体のエネルギー需要を満たすために酸化することができ、肝臓が糖質から生成したトリグリセリドを貯蔵することによって過剰なグルコースから身体を保護することであるが、糖質からの脂質合成のほとんどが脂肪組織自体で起こることを示唆する証拠もある。体のさまざまな部位にある脂肪組織は、生化学的プロフィールが異なっている。正常な状態では、脂肪組織は空腹と食事に関するフィードバックを脳に与える。
マウス
マウスには8つの主要な脂肪腺があり、そのうち4つは腹腔内にある。一対の生殖腺脂肪床はメスでは子宮と卵巣、オスでは精巣上体と精巣に付着している。一対の後腹膜脂肪床は腹部の背側壁に沿って存在し、腎臓を取り囲んでおり、巨大になると骨盤にまで及ぶ。 腸間膜は腸と卵膜(胃と脾臓の近くで発生する)を支える糊のような網を形成し、 -巨大になると -腹部まで伸びる。 腸間膜および卵膜の両デポには、それぞれリンパ節および乳白色斑として多くのリンパ組織が取り込まれている。
この2つの表在性脂肪層は、後肢の上肢節の前方(皮膚の下)にある一対の鼠径部脂肪層と、肩甲骨の背稜間の皮膚の下にある、白色脂肪組織の領域に隣接する褐色脂肪組織の内側にある一対の肩甲下脂肪層である。 このデポの褐色脂肪組織の層は、しばしば白色脂肪組織の「すりガラス」で覆われている;これらの2つのタイプの脂肪(褐色と白色)の区別が難しいこともある。 鼠径リンパ節は鼠径リンパ節群を包んでいる。 マイナーデポには、心臓を包む心膜と、膝の後ろの主要な筋肉の間にある一対の膝窩デポがあり、それぞれに大きなリンパ節が1つずつある。 マウスのすべてのデポの中で、生殖腺デポは最も大きく、最も容易に剥離可能であり、剥離可能な脂肪の約30%を占める。
肥満
肥満者において、腹部から下方に垂れ下がる余分な脂肪組織はパンニクルスと呼ばれる。パンニクルスは病的肥満者の手術を複雑にする。重度の肥満者が大量の脂肪を失った場合(胃バイパス手術の一般的な結果)、文字通り「皮膚のエプロン」として残ることがある。 肥満は、運動療法、食事療法、行動療法によって治療される。再建手術は治療の一側面である。
内臓脂肪===
内臓脂肪または腹部脂肪(臓器脂肪または腹腔内脂肪とも呼ばれる)は腹腔内にあり、臓器(胃、肝臓、腸、腎臓など)の間に詰まっている。 内臓脂肪は皮膚の下にある皮下脂肪や骨格筋に散在する筋肉内脂肪とは異なる。太ももや臀部のような下半身の脂肪は皮下脂肪であり、一貫した間隔のない組織であるのに対し、腹部の脂肪はほとんどが内臓脂肪であり、半流動体である。内臓脂肪は腸間膜、精巣上体白色脂肪組織(EWAT)、腎周囲デポを含むいくつかの脂肪腺から構成されている。内臓脂肪はしばしばcm2単位の面積で表される(VFA、内臓脂肪面積)。
内臓脂肪の過剰は腹部肥満、または腹部が過度に突出する「腹部脂肪」として知られている。体積指数(BVI)のような新しい開発は、腹部体積と腹部脂肪を測定するために特別に設計されている。過剰な内臓脂肪は、|2型糖尿病、インスリン抵抗性、炎症性疾患、およびその他の肥満関連疾患とも関連している。 同様に、頸部脂肪(または頸部脂肪組織)の蓄積は死亡率と関連することが示されている。いくつかの研究では、内臓脂肪は単純な体格測定から予測でき、肥満度やウエスト周囲径よりも正確に死亡率を予測することが示唆されている。
男性は性ホルモンの違いにより腹部に脂肪が蓄積しやすい。エストロゲン(女性ホルモン)により、女性の場合、脂肪は臀部、大腿部、臀部に蓄積される。女性が閉経を迎え、卵巣から分泌されるエストロゲンが減少すると、脂肪は臀部、臀部、大腿部から腰部に移動し、その後脂肪は腹部に蓄積される。
内臓脂肪はコルチゾールの過剰レベルによって引き起こされる可能性がある。少なくとも週10MET時間の有酸素運動は、代謝関連疾患のない人の内臓脂肪減少につながる。レジスタンストレーニングとカロリー制限も内臓脂肪を減少させるが、その効果は累積しないことがある。運動と低カロリー食の両方が内臓脂肪の減少を引き起こすが、運動は総脂肪に対して内臓脂肪により大きな影響を及ぼす。高強度の運動は、腹部総脂肪を効果的に減少させる方法の一つである。運動と組み合わせたエネルギー制限食は、総体脂肪および内臓脂肪組織と皮下脂肪組織の比率を減少させ、皮下脂肪よりも内臓脂肪を優先的に動員することを示唆している。
心外膜脂肪
心外膜脂肪組織(EAT)は、心臓の周囲に沈着する内臓脂肪の特殊な形態であり、様々な生理活性分子を生成する代謝的に活性な器官であることが判明しており、心機能に重大な影響を及ぼす可能性がある。EATと皮下脂肪を比較すると顕著な成分の違いが観察され、脂肪細胞の機能と代謝に対する蓄積脂肪酸の場所特異的な影響が示唆されている。
Subcutaneous fat
Most of the remaining nonvisceral fat is found just below the skin in a region called the hypodermis. This subcutaneous fat is not related to many of the classic obesity-related pathologies, such as heart disease, cancer, and stroke, and some evidence even suggests it might be protective. The typically female (or gynecoid) pattern of body fat distribution around the hips, thighs, and buttocks is subcutaneous fat, and therefore poses less of a health risk compared to visceral fat.
Like all other fat organs, subcutaneous fat is an active part of the endocrine system, secreting the hormones leptin and resistin.
The relationship between the subcutaneous adipose layer and total body fat in a person is often modelled by using regression equations. The most popular of these equations was formed by Durnin and Wormersley, who rigorously tested many types of skinfold, and, as a result, created two formulae to calculate the body density of both men and women. These equations present an inverse correlation between skinfolds and body density—as the sum of skinfolds increases, the body density decreases.
Factors such as sex, age, population size or other variables may make the equations invalid and unusable, and, 2012年現在[update], Durnin and Wormersley's equations remain only estimates of a person's true level of fatness. New formulae are still being created.
Marrow fat
Marrow fat, also known as marrow adipose tissue (MAT), is a poorly understood adipose depot that resides in the bone and is interspersed with hematopoietic cells as well as bony elements. The adipocytes in this depot are derived from mesenchymal stem cells (MSC) which can give rise to fat cells, bone cells as well as other cell types. The fact that MAT increases in the setting of calorie restriction/ anorexia is a feature that distinguishes this depot from other fat depots. Exercise regulates MAT, decreasing MAT quantity and diminishing the size of marrow adipocytes. The exercise regulation of marrow fat suggests that it bears some physiologic similarity to other white adipose depots. Moreover, increased MAT in obesity further suggests a similarity to white fat depots.
Ectopic fat
Ectopic fat is the storage of triglycerides in tissues other than adipose tissue, that are supposed to contain only small amounts of fat, such as the liver, skeletal muscle, heart, and pancreas. This can interfere with cellular functions and hence organ function and is associated with insulin resistance in type-2 diabetes. It is stored in relatively high amounts around the organs of the abdominal cavity, but is not to be confused with visceral fat.
The specific cause for the accumulation of ectopic fat is unknown. The cause is likely a combination of genetic, environmental, and behavioral factors that are involved in excess energy intake and decreased physical activity. Substantial weight loss can reduce ectopic fat stores in all organs and this is associated with an improvement of the function of those organs.
In the latter case, non-invasive weight loss interventions like diet or exercise can decrease ectopic fat (particularly in heart and liver) in overweight or obese children and adults.
Physiology
Free fatty acids (FFAs) are liberated from lipoproteins by lipoprotein lipase (LPL) and enter the adipocyte, where they are reassembled into triglycerides by esterifying them onto glycerol.
There is a constant flux of FFAs entering and leaving adipose tissue. The net direction of this flux is controlled by insulin and leptin—if insulin is elevated, then there is a net inward flux of FFA, and only when insulin is low can FFA leave adipose tissue. Insulin secretion is stimulated by high blood sugar, which results from consuming carbohydrates.
In humans, lipolysis (hydrolysis of triglycerides into free fatty acids) is controlled through the balanced control of lipolytic B-adrenergic receptors and a2A-adrenergic receptor-mediated antilipolysis.
Fat cells have an important physiological role in maintaining triglyceride and free fatty acid levels, as well as determining insulin resistance. Abdominal fat has a different metabolic profile—being more prone to induce insulin resistance. This explains to a large degree why central obesity is a marker of impaired glucose tolerance and is an independent risk factor for cardiovascular disease (even in the absence of diabetes mellitus and hypertension). Studies of female monkeys at Wake Forest University (2009) discovered that individuals with higher stress have higher levels of visceral fat in their bodies. This suggests a possible cause-and-effect link between the two, wherein stress promotes the accumulation of visceral fat, which in turn causes hormonal and metabolic changes that contribute to heart disease and other health problems.
Recent advances in biotechnology have allowed for the harvesting of adult stem cells from adipose tissue, allowing stimulation of tissue regrowth using a patient's own cells. In addition, adipose-derived stem cells from both human and animals reportedly can be efficiently reprogrammed into induced pluripotent stem cells without the need for feeder cells. The use of a patient's own cells reduces the chance of tissue rejection and avoids ethical issues associated with the use of human embryonic stem cells. A growing body of evidence also suggests that different fat depots (i.e. abdominal, omental, pericardial) yield adipose-derived stem cells with different characteristics. These depot-dependent features include proliferation rate, immunophenotype, differentiation potential, gene expression, as well as sensitivity to hypoxic culture conditions. Oxygen levels seem to play an important role on the metabolism and in general the function of adipose-derived stem cells.
Adipose tissue is a major peripheral source of aromatase in both males and females, contributing to the production of estradiol.
Adipose derived hormones include:
- Adiponectin
- Resistin
- Plasminogen activator inhibitor-1 (PAI-1)
- TNFα
- IL-6
- Leptin
- Estradiol (E2)
Adipose tissues also secrete a type of cytokines (cell-to-cell signalling proteins) called adipokines (adipose cytokines), which play a role in obesity-associated complications. Perivascular adipose tissue releases adipokines such as adiponectin that affect the contractile function of the vessels that they surround.
Brown fat
Brown fat or brown adipose tissue (BAT) is a specialized form of adipose tissue important for adaptive thermogenesis in humans and other mammals. BAT can generate heat by "uncoupling" the respiratory chain of oxidative phosphorylation within mitochondria through tissue-specific expression of uncoupling protein 1 (UCP1). BAT is primarily located around the neck and large blood vessels of the thorax, where it may effectively act in heat exchange. BAT is robustly activated upon cold exposure by the release of catecholamines from sympathetic nerves that results in UCP1 activation. Nearly half of the nerves present in adipose tissue are sensory neurons connected to the dorsal root ganglia.
BAT activation may also occur in response to overfeeding. UCP1 activity is stimulated by long chain fatty acids that are produced subsequent to β-adrenergic receptor activation. UCP1 is proposed to function as a fatty acid proton symporter, although the exact mechanism has yet to be elucidated. In contrast, UCP1 is inhibited by ATP, ADP, and GTP.
Attempts to simulate this process pharmacologically have so far been unsuccessful. Techniques to manipulate the differentiation of "brown fat" could become a mechanism for weight loss therapy in the future, encouraging the growth of tissue with this specialized metabolism without inducing it in other organs. A review on the eventual therapeutic targeting of brown fat to treat human obesity was published by Samuelson and Vidal-Puig in 2020.
Until recently, brown adipose tissue in humans was thought to be primarily limited to infants, but new evidence has overturned that belief. Metabolically active tissue with temperature responses similar to brown adipose was first reported in the neck and trunk of some human adults in 2007, and the presence of brown adipose in human adults was later verified histologically in the same anatomical regions.
Beige fat and WAT browning
Browning of WAT, also referred to as "beiging", occurs when adipocytes within WAT depots develop features of BAT. Beige adipocytes take on a multilocular appearance (containing several lipid droplets) and increase expression of uncoupling protein 1 (UCP1). In doing so, these normally energy-storing adipocytes become energy-releasing adipocytes.
The calorie-burning capacity of brown and beige fat has been extensively studied as research efforts focus on therapies targeted to treat obesity and diabetes. The drug 2,4-dinitrophenol, which also acts as a chemical uncoupler similarly to UCP1, was used for weight loss in the 1930s. However, it was quickly discontinued when excessive dosing led to adverse side effects including hyperthermia and death. β3 agonists, like CL316,243, have also been developed and tested in humans. However, the use of such drugs has proven largely unsuccessful due to several challenges, including varying species receptor specificity and poor oral bioavailability.
Cold is a primary regulator of BAT processes and induces WAT browning. Browning in response to chronic cold exposure has been well documented and is a reversible process. A study in mice demonstrated that cold-induced browning can be completely reversed in 21 days, with measurable decreases in UCP1 seen within a 24-hour period. A study by Rosenwald et al. revealed that when the animals are re-exposed to a cold environment, the same adipocytes will adopt a beige phenotype, suggesting that beige adipocytes are retained.
Transcriptional regulators, as well as a growing number of other factors, regulate the induction of beige fat. Four regulators of transcription are central to WAT browning and serve as targets for many of the molecules known to influence this process. These include peroxisome proliferator-activated receptor gamma (PPARγ), PRDM16, peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α), and Early B-Cell Factor-2 (EBF2).
The list of molecules that influence browning has grown in direct proportion to the popularity of this topic and is constantly evolving as more knowledge is acquired. Among these molecules are irisin and fibroblast growth factor 21 (FGF21), which have been well-studied and are believed to be important regulators of browning. Irisin is secreted from muscle in response to exercise and has been shown to increase browning by acting on beige preadipocytes. FGF21, a hormone secreted mainly by the liver, has garnered a great deal of interest after being identified as a potent stimulator of glucose uptake and a browning regulator through its effects on PGC-1α. It is increased in BAT during cold exposure and is thought to aid in resistance to diet-induced obesity FGF21 may also be secreted in response to exercise and a low protein diet, although the latter has not been thoroughly investigated. Data from these studies suggest that environmental factors like diet and exercise may be important mediators of browning. In mice, it was found that beiging can occur through the production of methionine-enkephalin peptides by type 2 innate lymphoid cells in response to interleukin 33.
Genomics and bioinformatics tools to study browning
Due to the complex nature of adipose tissue and a growing list of browning regulatory molecules, great potential exists for the use of bioinformatics tools to improve study within this field. Studies of WAT browning have greatly benefited from advances in these techniques, as beige fat is rapidly gaining popularity as a therapeutic target for the treatment of obesity and diabetes.
DNA microarray is a bioinformatics tool used to quantify expression levels of various genes simultaneously, and has been used extensively in the study of adipose tissue. One such study used microarray analysis in conjunction with Ingenuity IPA software to look at changes in WAT and BAT gene expression when mice were exposed to temperatures of 28 and 6 °C. The most significantly up- and downregulated genes were then identified and used for analysis of differentially expressed pathways. It was discovered that many of the pathways upregulated in WAT after cold exposure are also highly expressed in BAT, such as oxidative phosphorylation, fatty acid metabolism, and pyruvate metabolism. This suggests that some of the adipocytes switched to a beige phenotype at 6 °C. Mössenböck et al. also used microarray analysis to demonstrate that insulin deficiency inhibits the differentiation of beige adipocytes but does not disturb their capacity for browning. These two studies demonstrate the potential for the use of microarray in the study of WAT browning.
RNA sequencing (RNA-Seq) is a powerful computational tool that allows for the quantification of RNA expression for all genes within a sample. Incorporating RNA-Seq into browning studies is of great value, as it offers better specificity, sensitivity, and a more comprehensive overview of gene expression than other methods. RNA-Seq has been used in both human and mouse studies in an attempt characterize beige adipocytes according to their gene expression profiles and to identify potential therapeutic molecules that may induce the beige phenotype. One such study used RNA-Seq to compare gene expression profiles of WAT from wild-type (WT) mice and those overexpressing Early B-Cell Factor-2 (EBF2). WAT from the transgenic animals exhibited a brown fat gene program and had decreased WAT specific gene expression compared to the WT mice. Thus, EBF2 has been identified as a potential therapeutic molecule to induce beiging.
Chromatin immunoprecipitation with sequencing (ChIP-seq) is a method used to identify protein binding sites on DNA and assess histone modifications. This tool has enabled examination of epigenetic regulation of browning and helps elucidate the mechanisms by which protein-DNA interactions stimulate the differentiation of beige adipocytes. Studies observing the chromatin landscapes of beige adipocytes have found that adipogenesis of these cells results from the formation of cell specific chromatin landscapes, which regulate the transcriptional program and, ultimately, control differentiation. Using ChIP-seq in conjunction with other tools, recent studies have identified over 30 transcriptional and epigenetic factors that influence beige adipocyte development.
Genetics
The thrifty gene hypothesis (also called the famine hypothesis) states that in some populations the body would be more efficient at retaining fat in times of plenty, thereby endowing greater resistance to starvation in times of food scarcity. This hypothesis, originally advanced in the context of glucose metabolism and insulin resistance, has been discredited by physical anthropologists, physiologists, and the original proponent of the idea himself with respect to that context, although according to its developer it remains "as viable as when [it was] first advanced" in other contexts.
In 1995, Jeffrey Friedman, in his residency at the Rockefeller University, together with Rudolph Leibel, Douglas Coleman et al. discovered the protein leptin that the genetically obese mouse lacked. Leptin is produced in the white adipose tissue and signals to the hypothalamus. When leptin levels drop, the body interprets this as a loss of energy, and hunger increases. Mice lacking this protein eat until they are four times their normal size.
Leptin, however, plays a different role in diet-induced obesity in rodents and humans. Because adipocytes produce leptin, leptin levels are elevated in the obese. However, hunger remains, and—when leptin levels drop due to weight loss—hunger increases. The drop of leptin is better viewed as a starvation signal than the rise of leptin as a satiety signal. However, elevated leptin in obesity is known as leptin resistance. The changes that occur in the hypothalamus to result in leptin resistance in obesity are currently the focus of obesity research.
Gene defects in the leptin gene (ob) are rare in human obesity. July 2010現在[update], only 14 individuals from five families have been identified worldwide who carry a mutated ob gene (one of which was the first ever identified cause of genetic obesity in humans)—two families of Pakistani origin living in the UK, one family living in Turkey, one in Egypt, and one in Austria—and two other families have been found that carry a mutated ob receptor. Others have been identified as genetically partially deficient in leptin, and, in these individuals, leptin levels on the low end of the normal range can predict obesity.
Several mutations of genes involving the melanocortins (used in brain signaling associated with appetite) and their receptors have also been identified as causing obesity in a larger portion of the population than leptin mutations.
Physical properties
Adipose tissue has a density of ~0.9 g/ml. Thus, a person with more adipose tissue will float more easily than a person of the same weight with more muscular tissue, since muscular tissue has a density of 1.06 g/ml.
Body fat meter
A body fat meter is a tool used to measure the body fat to weight ratio in the human body. Different meters use various methods to determine the ratio. They tend to under-read body fat percentage.
In contrast with clinical tools, one relatively inexpensive type of body fat meter uses the principle of bioelectrical impedance analysis (BIA) in order to determine an individual's body fat percentage. To achieve this, the meter passes a small, harmless, electric current through the body and measures the resistance, then uses information on the person's weight, height, age, and sex to calculate an approximate value for the person's body fat percentage. The calculation measures the total volume of water in the body (lean tissue and muscle contain a higher percentage of water than fat), and estimates the percentage of fat based on this information. The result can fluctuate several percentage points depending on what has been eaten and how much water has been drunk before the analysis.
Before bioelectrical impedance analysis machines were developed, there were many different ways in analyzing body composition such as skin fold methods using calipers, underwater weighing, whole body air displacement plethysmography (ADP) and DXA.
Animal studies
Within the fat (adipose) tissue of CCR2 deficient mice, there is an increased number of eosinophils, greater alternative Macrophage activation, and a propensity towards type 2 cytokine expression. Furthermore, this effect was exaggerated when the mice became obese from a high fat diet.
ギャラリー
-
皮膚の断面図(拡大)
-
パラフィン切片の白色脂肪組織
-
体脂肪計の電子機器
こちらも参照
- Adipose differentiation-related protein/ja
- Adipocytes/ja
- Apelin/ja
- Bioelectrical impedance analysis/ja – 体脂肪率を測定する方法である。
- Blubber/ja – 一部の海生哺乳類に見られる脂肪組織で、非常に厚い。
- Body fat percentage/ja
- Cellulite/ja
- Lipolysis/ja
- Lipodystrophy/ja
- Human fat/ja 伝統医学で医薬品として使用されている
- 肥満
- Starvation/ja
- Steatosis/ja (脂肪変化、脂肪変性、脂肪変性とも呼ばれる。)
- Stem cells/ja
- Subcutaneous fat/ja
- Bariatrics/ja
- Classification of obesity/ja
- Classification of childhood obesity/ja
- EPODE International Network/ja, 世界最大の肥満防止ネットワーク
- World Fit/ja 米国オリンピック委員会(USOC)および米国オリンピアン・パラリンピアン協会(USOP)のプログラムである。
- Obesity and walking/ja
- Social stigma of obesity/ja
さらに読む
- Stock MJ, Cinti S (2003). "Adipose Tissue / Structure and Function of Brown Adipose Tissue". Encyclopedia of Food Sciences and Nutrition. pp. 29–34. doi:10.1016/B0-12-227055-X/00008-0. ISBN 978-0-12-227055-0.
- Vernon RG, Flint DJ (2003). "Adipose Tissue / Structure and Function of White Adipose Tissue". Encyclopedia of Food Sciences and Nutrition. pp. 23–29. doi:10.1016/B0-12-227055-X/00007-9. ISBN 978-0-12-227055-0.
外部リンク
- Adipose tissue at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- 脂肪組織の顕微鏡写真