ニコチンアミドリボシド
Nicotinamide riboside/ja
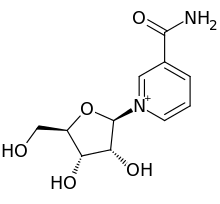
ニコチンアミドリボシド(NR、SR647)は、ピリジン-ヌクレオシドの一種で、ビタミンB3の一種である。ニコチンアミドアデニンジヌクレオチド、またはNAD+の前駆体として機能する。
| |

| |
| Names | |
|---|---|
| IUPAC name
3-Carbamoyl-1-(β-D-ribofuranosyl)pyridin-1-ium
| |
| Systematic IUPAC name
3-Carbamoyl-1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyridin-1-ium | |
| Other names
1-(β-D-Ribofuranosyl)nicotinamide; N-Ribosylnicotinamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H15N2O5+ | |
| Molar mass | 255.25 g/mol |
化学
ニコチンアミドリボシドの分子量が255.25g/molであるのに対し、塩化物塩の分子量は290.70g/molである。そのため、100 mgの塩化ニコチンアミドリボシドは88 mgのニコチンアミドリボシドを提供する。
歴史
ニコチンアミドリボシド(NR)は、細菌と真核生物の両方においてサルベージNAD合成に関与するNAD前駆体として同定されている。 細菌では、1944年にインフルエンザ菌の培養に必要な増殖因子として初めて報告され、インフルエンザ菌は増殖するためにX因子(ヘミン)とV因子(NAD)の両方を必要とすることが同定された。 血液から精製されたV因子は、ニコチンアミドアデニンジヌクレオチド(NAD+)、NMN、NRの3つの形態で存在することが示された。 NRはインフルエンザ菌を最も急速に増殖させた化合物であった。
インフルエンザ菌はニコチン酸(NA)、ニコチンアミド(NAM)、トリプトファン(Trp)やアスパラギン酸(Asp)のようなアミノ酸では増殖できない。 インフルエンザ菌は、環境中の他の細胞からのNAD前駆体のサルベージに完全に依存している。
真核生物におけるNAD前駆体としてのニコチンアミドリボシド(NR)の同定は、ペラグラの研究から発展した。ペラグラはNAD+欠乏に関連する最初の疾患であった。1914年にジョセフ・ゴールドバーガーによって栄養欠乏と関連づけられ、1937年にはコンラッド・エルヴェヘムによってナイアシン(ビタミンB3)の欠乏と関連づけられた。 ペラグラの症例ではNAD+(当時は補酵素Iと呼ばれていた)が極端に低下していることが示され、NAとNAMがNAD+レベルを回復させる分子前駆体として同定された。ペラグラは現在、NAD+の深刻な慢性的枯渇として理解されており、食事療法によって治療することができる。
Subsequent studies of NAD+ metabolism have identified regulatory pathways used by cells and tissues to maintain NAD+ availability. NAD+ and its precursors nicotinic acid (NA) and nicotinamide (NAM) have been shown to be vital cofactors in cellular oxidation/reduction reactions and ATP synthesis. Classic NAD+ synthesis pathways characterized in eukaryotes include an eight-step de novo pathway from Trp and two pathways using the NAD+ precursors NA and NAM: a three-step NA-based pathway known as the Preiss-Handler pathway; and an NAM-based pathway involving the enzyme Nicotinamide phosphoribosyltransferase (NAMPT) and the formation of nicotinamide mononucleotide (NMN).
In 2004, a previously unknown pathway was reported when nicotinamide riboside (NR) was identified as an additional NAD+ precursor in eukaryotes. NR is now recognized as a form of vitamin B3 which can be found in both cow and human milk. Once internalized into a cell, NR is rapidly phosphorylated by the activity of nicotinamide riboside kinase enzymes (NRK1 and NRK2) to form nicotinamide mononucleotide (NMN), bypassing the previously known biosynthetic routes to NAD+ production. NMN is then converted to NAD+ by NMN-adenylyltransferase (NMNAT).
Research in mammals indicates that NRK1 is a cytosolic protein, encoded by the Nmrk1 gene. It is found in most tissues but predominantly in the liver and kidney. The NRK2 protein may be related to muscle tissue including cardiac muscle. It is encoded by the Nmrk2 gene and appears to be more highly expressed in cases of metabolic stress or cellular damage. Since different types of tissues display differing concentrations of NR and NRKs, it is likely that NR utilization will vary in different tissues.
Metabolic studies indicate that NAD+, once considered a stable molecule, is continuously turned over and used, requiring tight regulation to maintain metabolic homeostasis. NR utilization in mammals may involve both exogenous dietary sources and endogenous salvage processes that recycle intermediates. NR metabolism and the interactions of different NAD+ pathways continue to be studied. The NAM and NR pathways involve an amide group and are referred to as ‘amidated’ pathways. The pathways for de novo synthesis from tryptophan and from NA salvage are ‘deamidated’ pathways, which share a rate-limiting amidation enzyme NADsynthase1 (NADSYN). Disruptions or imbalances in NAD+ metabolism have been observed in many disease conditions, and the possibility of restoring NAD+ levels by administering NAD+ precursors is an area of interest for researchers.
Biosynthesis
Nicotinamide riboside (NR) is now known to be an NAD+ precursor, involved in the biosynthetic pathways that convert B3 vitamins into NAD+. NAD+ is primarily synthesized in mammals de novo from tryptophan, through the Priess-Handler pathway from nicotinic acid (NA) or via a salvage pathway from nicotinamide (NAM).
Nicotinamide riboside (NR) is utilized through an additional pathway involving phosphorylation by the nicotinamide riboside kinase enzymes (NRK1 and NRK2). In yeasts, NR has also been shown to be degraded by the nucleosidases Pnp1, Urh1 and Meu1, before being converted to NAD⁺ via the Preiss-Handler pathway and the action of the nicotinamidase Pnc1.
Commercialization
ChromaDex licensed patents in July 2012, and began to develop a process to bring NR to market as TruNiagen. ChromaDex has been in a patent dispute with Elysium Health over the rights to nicotinamide riboside supplements since 2016.
Safety designations
In 2016, the U.S. Food and Drug Administration (FDA) has granted Generally recognized as safe (GRAS) status to ChromaDex for its preparation of nicotinamide riboside chloride (NRC, Niagen™). It was designated a new dietary ingredient (NDI) for use in dietary supplements by the U.S. Food and Drug Administration in 2015 and 2017. It was listed in Health Canada's Licensed Natural Health Products Database (LNHPD) in 2018. The European Union has granted NRC a "New dietary ingredient" designation as a novel food pursuant to Regulation (EU) 2015/2283, as of 2019. It was authorized for use in food supplements by the EU in 2020. The EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA) considered it as safe as pure nicotinamide for use in food for special medical purposes (FSMP) and total diet replacement for weight control (TDRWC) in adults as of 2021 but noted that further investigation would be required to establish safety for some other types of use. The Australian government has given nicotinamide riboside chloride a positive listing under the compositional guidelines of its Therapeutic Goods Administration (TGA).