Maltose: Difference between revisions
Created page with "<languages /> <translate> {{chembox | Verifiedfields = changed | Watchedfields = removed | verifiedrevid = 47716923 | ImageFile = Maltose2.svg | ImageSize = | ImageName = α-Maltose | ImageCaption = α-Maltose | ImageFile1 = Maltose structure.svg | ImageSize1 = | ImageName1 = β-Maltose | ImageCaption1 = β-Maltose | IUPACName = 4-''O''-α-<small>D</small>-Glucopyranosyl-<small>D</small>-glucose | OtherNames = | SystematicName..." |
Marked this version for translation |
||
| Line 1: | Line 1: | ||
<languages /> | <languages /> | ||
<translate> | <translate> | ||
<!--T:1--> | |||
{{chembox | {{chembox | ||
| Verifiedfields = changed | | Verifiedfields = changed | ||
| Line 63: | Line 64: | ||
[[Image:Amylase reaction.png|thumb|right|260px|Amylase reaction consisting of hydrolyzing amylose, producing maltose]] | [[Image:Amylase reaction.png|thumb|right|260px|Amylase reaction consisting of hydrolyzing amylose, producing maltose]] | ||
<!--T:2--> | |||
'''Maltose''' ({{IPAc-en|ˈ|m|ɔː|l|t|oʊ|s}} or {{IPAc-en|ˈ|m|ɔː|l|t|oʊ|z}}), also known as '''maltobiose''' or '''malt sugar''', is a [[disaccharide]] formed from two units of [[glucose]] joined with an α(1→4) [[glycosidic bond|bond]]. In the [[isomer]] [[isomaltose]], the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the [[amylose]] [[homologous series]], the key structural motif of [[starch]]. When [[beta-amylase]] breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in [[germination|germinating]] seeds, which is why it was named after [[malt]]. Unlike [[sucrose]], it is a [[reducing sugar]]. | '''Maltose''' ({{IPAc-en|ˈ|m|ɔː|l|t|oʊ|s}} or {{IPAc-en|ˈ|m|ɔː|l|t|oʊ|z}}), also known as '''maltobiose''' or '''malt sugar''', is a [[disaccharide]] formed from two units of [[glucose]] joined with an α(1→4) [[glycosidic bond|bond]]. In the [[isomer]] [[isomaltose]], the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the [[amylose]] [[homologous series]], the key structural motif of [[starch]]. When [[beta-amylase]] breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in [[germination|germinating]] seeds, which is why it was named after [[malt]]. Unlike [[sucrose]], it is a [[reducing sugar]]. | ||
<!--T:3--> | |||
== History == | == History == | ||
Maltose was discovered by [[Augustin-Pierre Dubrunfaut]], although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer [[Cornelius O'Sullivan]]. Its name comes from [[malt]], combined with the suffix '[[-ose]]' which is used in names of sugars. | Maltose was discovered by [[Augustin-Pierre Dubrunfaut]], although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer [[Cornelius O'Sullivan]]. Its name comes from [[malt]], combined with the suffix '[[-ose]]' which is used in names of sugars. | ||
<!--T:4--> | |||
== Structure and nomenclature == | == Structure and nomenclature == | ||
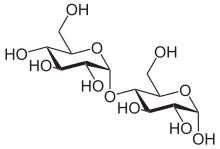
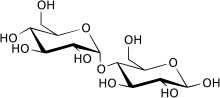
Carbohydrates are generally divided into [[monosaccharide]]s, [[oligosaccharide]]s, and [[polysaccharide]]s depending on the number of sugar subunits. Maltose, with two sugar units, is a disaccharide, which falls under oligosaccharides. Glucose is a [[hexose]]: a monosaccharide containing six carbon atoms. The two glucose units are in the [[pyranose]] form and are joined by an [[glycosidic bond|O-glycosidic bond]], with the first carbon (C<sub>1</sub>) of the first [[glucose]] linked to the fourth carbon (C<sub>4</sub>) of the second [[glucose]], indicated as (1→4). The link is characterized as α because the glycosidic bond to the anomeric carbon (C<sub>1</sub>) is in the opposite plane from the {{chem|C|H|2|O|H}} [[substituent]] in the same ring (C<sub>6</sub> of the first glucose). If the glycosidic bond to the anomeric carbon (C<sub>1</sub>) were in the same plane as the {{chem|C|H|2|O|H}} substituent, it would be classified as a '''β(1→4)''' bond, and the resulting molecule would be [[cellobiose]]. The anomeric carbon (C<sub>1</sub>) of the second glucose molecule, which is not involved in a glycosidic bond, could be either an α- or β-anomer depending on the bond direction of the attached hydroxyl group relative to the {{chem|C|H|2|O|H}} substituent of the same ring, resulting in either α-maltose or β-maltose. | Carbohydrates are generally divided into [[monosaccharide]]s, [[oligosaccharide]]s, and [[polysaccharide]]s depending on the number of sugar subunits. Maltose, with two sugar units, is a disaccharide, which falls under oligosaccharides. Glucose is a [[hexose]]: a monosaccharide containing six carbon atoms. The two glucose units are in the [[pyranose]] form and are joined by an [[glycosidic bond|O-glycosidic bond]], with the first carbon (C<sub>1</sub>) of the first [[glucose]] linked to the fourth carbon (C<sub>4</sub>) of the second [[glucose]], indicated as (1→4). The link is characterized as α because the glycosidic bond to the anomeric carbon (C<sub>1</sub>) is in the opposite plane from the {{chem|C|H|2|O|H}} [[substituent]] in the same ring (C<sub>6</sub> of the first glucose). If the glycosidic bond to the anomeric carbon (C<sub>1</sub>) were in the same plane as the {{chem|C|H|2|O|H}} substituent, it would be classified as a '''β(1→4)''' bond, and the resulting molecule would be [[cellobiose]]. The anomeric carbon (C<sub>1</sub>) of the second glucose molecule, which is not involved in a glycosidic bond, could be either an α- or β-anomer depending on the bond direction of the attached hydroxyl group relative to the {{chem|C|H|2|O|H}} substituent of the same ring, resulting in either α-maltose or β-maltose. | ||
<!--T:5--> | |||
An [[isomer]] of maltose is [[isomaltose]]. This is similar to maltose but instead of a bond in the α(1→4) position, it is in the α(1→6) position, the same bond that is found at the branch points of [[glycogen]] and [[amylopectin]]. | An [[isomer]] of maltose is [[isomaltose]]. This is similar to maltose but instead of a bond in the α(1→4) position, it is in the α(1→6) position, the same bond that is found at the branch points of [[glycogen]] and [[amylopectin]]. | ||
<!--T:6--> | |||
== Properties == | == Properties == | ||
Like glucose, maltose is a [[reducing sugar]], because the ring of one of the two glucose units can open to present a free [[aldehyde]] group; the other one cannot because of the nature of the glycosidic bond. Maltose can be broken down to glucose by the [[maltase]] enzyme, which catalyses the hydrolysis of the glycosidic bond. | Like glucose, maltose is a [[reducing sugar]], because the ring of one of the two glucose units can open to present a free [[aldehyde]] group; the other one cannot because of the nature of the glycosidic bond. Maltose can be broken down to glucose by the [[maltase]] enzyme, which catalyses the hydrolysis of the glycosidic bond. | ||
<!--T:7--> | |||
Maltose in aqueous solution exhibits [[mutarotation]], because the α and β isomers that are formed by the different conformations of the anomeric carbon have different [[specific rotation]]s, and in aqueous solutions, these two forms are in equilibrium. Maltose can easily be detected by the Woehlk test or Fearon's test on methylamine. | Maltose in aqueous solution exhibits [[mutarotation]], because the α and β isomers that are formed by the different conformations of the anomeric carbon have different [[specific rotation]]s, and in aqueous solutions, these two forms are in equilibrium. Maltose can easily be detected by the Woehlk test or Fearon's test on methylamine. | ||
<!--T:8--> | |||
It has a sweet taste, but is only about 30–60% as sweet as sugar, depending on the concentration. | It has a sweet taste, but is only about 30–60% as sweet as sugar, depending on the concentration. | ||
<!--T:9--> | |||
== Sources and absorption == | == Sources and absorption == | ||
[[Image:Maltose syrup.jpg|thumb|right|Maltose syrup]] | [[Image:Maltose syrup.jpg|thumb|right|Maltose syrup]] | ||
Maltose is a [[malt]] component, a substance obtained when the grain is softened in water and germinates. It is also present in highly variable quantities in partially hydrolyzed starch products like [[maltodextrin]], [[corn syrup]] and acid-thinned starch. | Maltose is a [[malt]] component, a substance obtained when the grain is softened in water and germinates. It is also present in highly variable quantities in partially hydrolyzed starch products like [[maltodextrin]], [[corn syrup]] and acid-thinned starch. | ||
<!--T:10--> | |||
Outside of plants, maltose is also (likely) found in [[sugarbag]]. | Outside of plants, maltose is also (likely) found in [[sugarbag]]. | ||
<!--T:11--> | |||
In humans, maltose is broken down by various maltase enzymes, providing two glucose molecules that can be [[Glucose metabolism|further processed]]: either broken down to provide energy, or stored as glycogen. The lack of the [[sucrase-isomaltase]] enzyme in humans causes [[sucrose intolerance]], but complete maltose intolerance is extremely rare because there are four different maltase enzymes. | In humans, maltose is broken down by various maltase enzymes, providing two glucose molecules that can be [[Glucose metabolism|further processed]]: either broken down to provide energy, or stored as glycogen. The lack of the [[sucrase-isomaltase]] enzyme in humans causes [[sucrose intolerance]], but complete maltose intolerance is extremely rare because there are four different maltase enzymes. | ||
<!--T:12--> | |||
==External links== | ==External links== | ||
*{{Commons category-inline}} | *{{Commons category-inline}} | ||
*[https://web.archive.org/web/20051124215824/http://www.elmhurst.edu/~chm/vchembook/546maltose.html Maltose], Elmhurst College Virtual Chembook. | *[https://web.archive.org/web/20051124215824/http://www.elmhurst.edu/~chm/vchembook/546maltose.html Maltose], Elmhurst College Virtual Chembook. | ||
<!--T:13--> | |||
{{Carbohydrates}} | {{Carbohydrates}} | ||
{{Sugar}} | {{Sugar}} | ||
<!--T:14--> | |||
{{二次利用|date=17 June 2023}} | {{二次利用|date=17 June 2023}} | ||
[[Category:Disaccharides]] | [[Category:Disaccharides]] | ||
Latest revision as of 20:02, 21 April 2024
 α-Maltose
| |
 β-Maltose
| |
| Names | |
|---|---|
| IUPAC name
4-O-α-D-Glucopyranosyl-D-glucose
| |
| Systematic IUPAC name
(3R,4R,5S,6R)-6-(hydroxymethyl)-5-{[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxane-2,3,4-triol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C12H22O11 | |
| Molar mass | 342.297 g·mol−1 |
| Appearance | White powder or crystals |
| Density | 1.54 g/cm3 |
| Melting point | 160 to 165 °C (320 to 329 °F; 433 to 438 K) (anhydrous) 102–103 °C (monohydrate) |
| 1.080 g/mL (20 °C) | |
Chiral rotation ([α]D)
|
+140.7° (H2O, c = 10) |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related
|
Sucrose Lactose Trehalose Cellobiose |

Maltose (/ˈmɔːltoʊs/ or /ˈmɔːltoʊz/), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar.
History
Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix '-ose' which is used in names of sugars.
Structure and nomenclature
Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the number of sugar subunits. Maltose, with two sugar units, is a disaccharide, which falls under oligosaccharides. Glucose is a hexose: a monosaccharide containing six carbon atoms. The two glucose units are in the pyranose form and are joined by an O-glycosidic bond, with the first carbon (C1) of the first glucose linked to the fourth carbon (C4) of the second glucose, indicated as (1→4). The link is characterized as α because the glycosidic bond to the anomeric carbon (C1) is in the opposite plane from the CH
2OH substituent in the same ring (C6 of the first glucose). If the glycosidic bond to the anomeric carbon (C1) were in the same plane as the CH
2OH substituent, it would be classified as a β(1→4) bond, and the resulting molecule would be cellobiose. The anomeric carbon (C1) of the second glucose molecule, which is not involved in a glycosidic bond, could be either an α- or β-anomer depending on the bond direction of the attached hydroxyl group relative to the CH
2OH substituent of the same ring, resulting in either α-maltose or β-maltose.
An isomer of maltose is isomaltose. This is similar to maltose but instead of a bond in the α(1→4) position, it is in the α(1→6) position, the same bond that is found at the branch points of glycogen and amylopectin.
Properties
Like glucose, maltose is a reducing sugar, because the ring of one of the two glucose units can open to present a free aldehyde group; the other one cannot because of the nature of the glycosidic bond. Maltose can be broken down to glucose by the maltase enzyme, which catalyses the hydrolysis of the glycosidic bond.
Maltose in aqueous solution exhibits mutarotation, because the α and β isomers that are formed by the different conformations of the anomeric carbon have different specific rotations, and in aqueous solutions, these two forms are in equilibrium. Maltose can easily be detected by the Woehlk test or Fearon's test on methylamine.
It has a sweet taste, but is only about 30–60% as sweet as sugar, depending on the concentration.
Sources and absorption

Maltose is a malt component, a substance obtained when the grain is softened in water and germinates. It is also present in highly variable quantities in partially hydrolyzed starch products like maltodextrin, corn syrup and acid-thinned starch.
Outside of plants, maltose is also (likely) found in sugarbag.
In humans, maltose is broken down by various maltase enzymes, providing two glucose molecules that can be further processed: either broken down to provide energy, or stored as glycogen. The lack of the sucrase-isomaltase enzyme in humans causes sucrose intolerance, but complete maltose intolerance is extremely rare because there are four different maltase enzymes.
External links
| この記事は、クリエイティブ・コモンズ・表示・継承ライセンス3.0のもとで公表されたウィキペディアの項目Maltose(17 June 2023編集記事参照)を素材として二次利用しています。 Item:Q22089 |