7-Keto-DHEA
7-Keto-DHEA
7-Keto-DHEA acetate
Names
IUPAC name
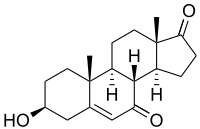
3β-Hydroxyandrost-5-ene-7,17-dione
Systematic IUPAC name
(3aS ,3bR ,7S ,9aR ,9bS ,11aS )-9a,11a-Dimethyl-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H -cyclopenta[a ]phenanthrene-1,4(3bH )-dione
Other names
7-Oxo-DHEA; 7-Ketodehydroepiandrosterone; 7-Oxodehydroepiandrosterone; 3β-Hydroxyandrost-5-ene-7,17-dione; Androst-5-en-3β-ol-7,17-dione
Identifiers
ChEBI
ChemSpider
UNII
InChI=1S/C19H26O3/c1-18-7-5-12(20)9-11(18)10-15(21)17-13-3-4-16(22)19(13,2)8-6-14(17)18/h10,12-14,17,20H,3-9H2,1-2H3/t12-,13-,14-,17-,18-,19-/m0/s1
Key: KPRGOTLNGIBVFL-GINZOMEDSA-N
InChI=1/C19H26O3/c1-18-7-5-12(20)9-11(18)10-15(21)17-13-3-4-16(22)19(13,2)8-6-14(17)18/h10,12-14,17,20H,3-9H2,1-2H3/t12-,13-,14-,17-,18-,19-/m0/s1
Key: KPRGOTLNGIBVFL-GINZOMEDBE
O=C2\C=C4/[C@]([C@H]3CC[C@@]1(C(=O)CC[C@H]1[C@H]23)C)(C)CC[C@H](O)C4
Properties
C 19 H 26 O 3
Molar mass
−1
7-Ketodehydroepiandrosterone (7-keto-DHEA , 7-oxo-DHEA ), also known as 7-oxoprasterone , is a steroid prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).
Pharmacodynamics 7-Keto-DHEA is not directly converted to testosterone or estrogen by the human body. Due to this fact, the suppliers of supplements have investigated it as a potentially more useful relative of the DHEA steroid when used as a supplementation, but the results are inconclusive.
Unsubstantiated health claims The benefits of 7-keto-DHEA supplementation are not definitively established. The health claims on potential weight loss benefits are not supported by solid evidence. The current evidence is mixed and limited by factors such as small sample sizes, short study durations, and a lack of diversity in the study populations. The safety profile of 7-keto-DHEA appears to be generally well-tolerated with a low side-effect profile, but changes in blood hormone parameters have been reported. Given these uncertainties, the potential benefits and safety of 7-keto-DHEA, particularly with long-term use, are not established. The US Food and Drug Administration (FDA) has not added 7-keto-DHEA to the list of bulk drug substances due to a lack of clinical evidence regarding its safety and efficacy.
In particular, 7-Keto-DHEA is marketed (also as 7-oxo-DHEA) to be more effective than DHEA for inducing heat production (thermogenesis ) to be used in weight loss: because dieting is usually accompanied by reduced resting metabolic rate , obese persons may benefit from using 7-keto-DHEA when dieting due to increased metabolic rate. Still, these claimed benefits are not supported by solid evidence.
7-Keto-DHEA has also been marketed by alternative medicine providers as a treatment of adrenal fatigue , a pseudo-scientific term with no scholarly basis.
Chemistry 7-keto-DHEA is a prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).
7-Keto-DHEA has a number of chemical names, including:
7-Ketodehydroepiandrosterone (7-keto-DHEA)
7-Oxodehydroepiandrosterone (7-oxo-DHEA)
7-Ketoprasterone
7-Oxoprasterone
3β-Hydroxyandrost-5-ene-7,17-dione
Androst-5-en-3β-ol-7,17-dione For the acetate ester:
3β-Acetoxyandrost-5-ene-7,17-dione
7-Oxo-dehydroepiandrosterone acetate (7-oxo-DHEA acetate)
3-Acetyl-7-oxo-dehydroepiandrosterone (3-acetyl-7-oxo-DHEA)
DHEA acetate-7-one
Δ5 -Androstene-3β-acetoxy-7,17-dione Note: "Keto" can be substituted for "oxo" in the above names.
Regulation The FDA has proposed that 7-Keto-DHEA be included among substances banned from use in compounded drugs .
7-Keto-DHEA may trigger positive tests for performance-enhancing drugs .
The World Anti-Doping Agency (WADA) lists 7-keto-DHEA as a prohibited anabolic agent.
See also
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown
この記事は、クリエイティブ・コモンズ・表示・継承ライセンス3.0 のもとで公表されたウィキペディアの項目7-Keto-DHEA (1 April 2024編集記事参照)を素材として二次利用しています。