コラーゲン
Collagen/ja
コラーゲン(/ˈkɒlədʒən/)は、主に軟骨、骨、腱、靭帯、皮膚などの身体の様々な結合組織に見られる細胞外マトリックスの主要な構造タンパク質である。結合組織の主成分として、哺乳類において最も豊富なタンパク質であり、全身のタンパク質含有量の25%から35%を占める。コラーゲンはアミノ酸が結合してコラーゲンヘリックスとして知られる細長い線維の三重らせんを形成している。ビタミンCはコラーゲンの合成に不可欠であり、ビタミンEはコラーゲンの産生を改善する。

コラーゲン組織は、ミネラルミネラリゼーションの程度によって、剛直(骨)かコンプライアント(腱)、あるいは剛直からコンプライアントへの勾配(軟骨)を持つ。コラーゲンは角膜、血管、腸、椎間板、歯の象牙質にも豊富に存在する。筋肉組織では、内膜の主要な構成成分として機能する。コラーゲンは筋肉組織の1~2%を占め、骨格筋組織の重量の6%を占める。線維芽細胞はコラーゲンを作り出す最も一般的な細胞である。食品や工業用に使用されるゼラチンは、熱、塩基性溶液、弱酸を用いて不可逆的に加水分解されたコラーゲンである。
語源
コラーゲンという名称は、"接着剤"を意味するギリシャ語κόλλα(kólla)と、「生成」を表す接尾辞-γέν(-gen)に由来する。
ヒトのタイプ
人体中のコラーゲンの90%以上はI型コラーゲンである。しかし、2011年現在、28種類のヒト・コラーゲンが同定、記述され、形成する構造によっていくつかのグループに分けられている。全てのタイプが少なくとも1つの三重らせんを含んでいる。この種類の多さは、コラーゲンの多様な機能性を示している。
- 線維性(タイプI、II、III、V、XI)
- 非線維性
最も一般的な5つのタイプがある:
- I型:皮膚、腱、血管系、臓器、骨(骨の有機部分の主成分)
- |II型: 軟骨(軟骨の主なコラーゲン成分)
- III型:網状(網状繊維の主成分)、I型と並んでよく見られる。
- IV型:基底膜(基底膜の上皮分泌層)を形成する。
- V型:細胞表面、毛髪、胎盤を形成する。
心臓
4つの心臓弁リングを含むコラーゲン質の心骨格は、組織学的に、弾力的かつ特異的に心筋に結合している。心骨格には、心室間隔と房室隔という心室の隔壁も含まれる。コラーゲンの心臓性能の測定への寄与は、要約すると心臓から放出される血圧の流体力学に対抗する連続的なねじり力を表す。心臓の上室と下室を仕切るコラーゲン構造は、典型的な生理学的手段によって血液と電気インパルスの両方を排除する不透過性の膜である。コラーゲンによるサポートがあれば、心房細動が心室細動に悪化することはない。コラーゲンは、平滑筋量とともに、さまざまな密度で層になっている。コラーゲンの質量、分布、年齢、密度はすべて、血液を前後に移動させるのに必要なコンプライアンスに寄与する。個々の心臓弁膜は、可変的な圧力の下で、特殊なコラーゲンによって形状に折り畳まれる。コラーゲン内のカルシウム沈着は、老化の自然な機能として徐々に起こる。コラーゲンマトリックス内の石灰化した点は、血液と筋肉の動くディスプレイの中でコントラストを示し、心臓画像技術の方法によって、本質的に血液の流入(心臓入力)と血液の流出(心臓出力)の比率を示すことができる。心臓を支えるコラーゲンの病理は結合組織病の範疇で理解されている。
骨移植
骨格は身体の構造を形成しているため、骨折や怪我の後でも強度を維持することが不可欠である。コラーゲンは三重らせん構造を持ち、非常に強い分子であるため、骨移植に使用される。骨格の構造的完全性を損なわないので、骨に使用するのに理想的である。コラーゲンの三重らせん構造は酵素による分解を防ぎ、細胞の接着を可能にし、細胞外マトリックスの適切な組み立てに重要である。
組織再生
コラーゲン・スキャフォールドは、スポンジ、薄いシート、ファイバーなど、組織再生に用いられている。コラーゲンは、孔構造、透過性、親水性、生体内での安定性など、組織再生に有利な特性を持つ。コラーゲンの足場はまた、骨芽細胞や線維芽細胞などの細胞の沈着を支持し、一度挿入されると成長が正常に進むように促進する。
Reconstructive surgical uses
Collagens are widely employed in the construction of artificial skin substitutes used in the management of severe burns and wounds. These collagens may be derived from bovine, equine, porcine, or even human sources; and are sometimes used in combination with silicones, glycosaminoglycans, fibroblasts, growth factors and other substances.
Wound healing
Collagen is one of the body's key natural resources and a component of skin tissue that can benefit all stages of wound healing. When collagen is made available to the wound bed, closure can occur. Wound deterioration, followed sometimes by procedures such as amputation, can thus be avoided.
Collagen is a natural product and is thus used as a natural wound dressing and has properties that artificial wound dressings do not have. It is resistant against bacteria, which is of vital importance in a wound dressing. It helps to keep the wound sterile, because of its natural ability to fight infection. When collagen is used as a burn dressing, healthy granulation tissue is able to form very quickly over the burn, helping it to heal rapidly.
Throughout the four phases of wound healing, collagen performs the following functions:
- Guiding function: Collagen fibers serve to guide fibroblasts. Fibroblasts migrate along a connective tissue matrix.
- Chemotactic properties: The large surface area available on collagen fibers can attract fibrogenic cells which help in healing.
- Nucleation: Collagen, in the presence of certain neutral salt molecules, can act as a nucleating agent causing formation of fibrillar structures.
- Hemostatic properties: Blood platelets interact with the collagen to make a hemostatic plug.
Basic research
Collagen is used in laboratory studies for cell culture, studying cell behavior and cellular interactions with the extracellular environment. Collagen is also widely used as a bioink for 3D bioprinting and biofabrication of 3D tissue models.
Biology
The collagen protein is composed of a triple helix, which generally consists of two identical chains (α1) and an additional chain that differs slightly in its chemical composition (α2). The amino acid composition of collagen is atypical for proteins, particularly with respect to its high hydroxyproline content. The most common motifs in the amino acid sequence of collagen are glycine-proline-X and glycine-X-hydroxyproline, where X is any amino acid other than glycine, proline or hydroxyproline. The average amino acid composition for fish and mammal skin is given.
| Amino acid | Abundance in mammal skin (residues/1000) |
Abundance in fish skin (residues/1000) |
|---|---|---|
| Glycine | 329 | 339 |
| Proline | 126 | 108 |
| Alanine | 109 | 114 |
| Hydroxyproline | 95 | 67 |
| Glutamic acid | 74 | 76 |
| Arginine | 49 | 52 |
| Aspartic acid | 47 | 47 |
| Serine | 36 | 46 |
| Lysine | 29 | 26 |
| Leucine | 24 | 23 |
| Valine | 22 | 21 |
| Threonine | 19 | 26 |
| Phenylalanine | 13 | 14 |
| Isoleucine | 11 | 11 |
| Hydroxylysine | 6 | 8 |
| Methionine | 6 | 13 |
| Histidine | 5 | 7 |
| Tyrosine | 3 | 3 |
| Cysteine | 1 | 1 |
| Tryptophan | 0 | 0 |
Synthesis
First, a three-dimensional stranded structure is assembled, with the amino acids glycine and proline as its principal components. This is not yet collagen but its precursor, procollagen. Procollagen is then modified by the addition of hydroxyl groups to the amino acids proline and lysine. This step is important for later glycosylation and the formation of the triple helix structure of collagen. Because the hydroxylase enzymes that perform these reactions require vitamin C as a cofactor, a long-term deficiency in this vitamin results in impaired collagen synthesis and scurvy. and lysyl hydroxylase. The reaction consumes one ascorbate molecule per hydroxylation.
The synthesis of collagen occurs inside and outside of the cell. The formation of collagen which results in fibrillary collagen (most common form) is discussed here. Meshwork collagen, which is often involved in the formation of filtration systems, is the other form of collagen. All types of collagens are triple helices, and the differences lie in the make-up of the alpha peptides created in step 2.
- Transcription of mRNA: About 44 genes are associated with collagen formation, each coding for a specific mRNA sequence, and typically have the "COL" prefix. The beginning of collagen synthesis begins with turning on genes which are associated with the formation of a particular alpha peptide (typically alpha 1, 2 or 3).
- Pre-pro-peptide formation: Once the final mRNA exits from the cell nucleus and enters into the cytoplasm, it links with the ribosomal subunits and the process of translation occurs. The early/first part of the new peptide is known as the signal sequence. The signal sequence on the N-terminal of the peptide is recognized by a signal recognition particle on the endoplasmic reticulum, which will be responsible for directing the pre-pro-peptide into the endoplasmic reticulum. Therefore, once the synthesis of new peptide is finished, it goes directly into the endoplasmic reticulum for post-translational processing. It is now known as preprocollagen.
- Pre-pro-peptide to pro-collagen: Three modifications of the pre-pro-peptide occur leading to the formation of the alpha peptide:
- The signal peptide on the N-terminal is removed, and the molecule is now known as propeptide (not procollagen).
- Hydroxylation of lysines and prolines on propeptide by the enzymes 'prolyl hydroxylase' and 'lysyl hydroxylase' (to produce hydroxyproline and hydroxylysine) occurs to aid cross-linking of the alpha peptides. This enzymatic step requires vitamin C as a cofactor. In scurvy, the lack of hydroxylation of prolines and lysines causes a looser triple helix (which is formed by three alpha peptides).
- Glycosylation occurs by adding either glucose or galactose monomers onto the hydroxyl groups that were placed onto lysines, but not on prolines.
- Once these modifications have taken place, three of the hydroxylated and glycosylated propeptides twist into a triple helix forming procollagen. Procollagen still has unwound ends, which will be later trimmed. At this point, the procollagen is packaged into a transfer vesicle destined for the Golgi apparatus.
- Golgi apparatus modification: In the Golgi apparatus, the procollagen goes through one last post-translational modification before being secreted out of the cell. In this step, oligosaccharides (not monosaccharides as in step 3) are added, and then the procollagen is packaged into a secretory vesicle destined for the extracellular space.
- Formation of tropocollagen: Once outside the cell, membrane bound enzymes known as collagen peptidases, remove the "loose ends" of the procollagen molecule. What is left is known as tropocollagen. Defects in this step produce one of the many collagenopathies known as Ehlers–Danlos syndrome. This step is absent when synthesizing type III, a type of fibrillar collagen.
- Formation of the collagen fibril: lysyl oxidase, an extracellular copper-dependent enzyme, produces the final step in the collagen synthesis pathway. This enzyme acts on lysines and hydroxylysines producing aldehyde groups, which will eventually undergo covalent bonding between tropocollagen molecules. This polymer of tropocollagen is known as a collagen fibril.
Amino acids
Collagen has an unusual amino acid composition and sequence:
- Glycine is found at almost every third residue.
- Proline makes up about 17% of collagen.
- Collagen contains two unusual derivative amino acids not directly inserted during translation. These amino acids are found at specific locations relative to glycine and are modified post-translationally by different enzymes, both of which require vitamin C as a cofactor.
- Hydroxyproline derived from proline
- Hydroxylysine derived from lysine – depending on the type of collagen, varying numbers of hydroxylysines are glycosylated (mostly having disaccharides attached).
Cortisol stimulates degradation of (skin) collagen into amino acids.
Collagen I formation
Most collagen forms in a similar manner, but the following process is typical for type I:
- Inside the cell
- Two types of alpha chains – alpha-1 and alpha 2, are formed during translation on ribosomes along the rough endoplasmic reticulum (RER). These peptide chains known as preprocollagen, have registration peptides on each end and a signal peptide.
- Polypeptide chains are released into the lumen of the RER.
- Signal peptides are cleaved inside the RER and the chains are now known as pro-alpha chains.
- Hydroxylation of lysine and proline amino acids occurs inside the lumen. This process is dependent on and consumes ascorbic acid (vitamin C) as a cofactor.
- Glycosylation of specific hydroxylysine residues occurs.
- Triple alpha helical structure is formed inside the endoplasmic reticulum from two alpha-1 chains and one alpha-2 chain.
- Procollagen is shipped to the Golgi apparatus, where it is packaged and secreted into extracellular space by exocytosis.
- Outside the cell
- Registration peptides are cleaved and tropocollagen is formed by procollagen peptidase.
- Multiple tropocollagen molecules form collagen fibrils, via covalent cross-linking (aldol reaction) by lysyl oxidase which links hydroxylysine and lysine residues. Multiple collagen fibrils form into collagen fibers.
- Collagen may be attached to cell membranes via several types of protein, including fibronectin, laminin, fibulin and integrin.
Synthetic pathogenesis
Vitamin C deficiency causes scurvy, a serious and painful disease in which defective collagen prevents the formation of strong connective tissue. Gums deteriorate and bleed, with loss of teeth; skin discolors, and wounds do not heal. Prior to the 18th century, this condition was notorious among long-duration military, particularly naval, expeditions during which participants were deprived of foods containing vitamin C.
An autoimmune disease such as lupus erythematosus or rheumatoid arthritis may attack healthy collagen fibers.
Many bacteria and viruses secrete virulence factors, such as the enzyme collagenase, which destroys collagen or interferes with its production.
Molecular structure
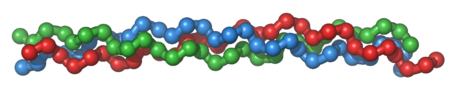
A single collagen molecule, tropocollagen, is used to make up larger collagen aggregates, such as fibrils. It is approximately 300 nm long and 1.5 nm in diameter, and it is made up of three polypeptide strands (called alpha peptides, see step 2), each of which has the conformation of a left-handed helix – this should not be confused with the right-handed alpha helix. These three left-handed helices are twisted together into a right-handed triple helix or "super helix", a cooperative quaternary structure stabilized by many hydrogen bonds. With type I collagen and possibly all fibrillar collagens, if not all collagens, each triple-helix associates into a right-handed super-super-coil referred to as the collagen microfibril. Each microfibril is interdigitated with its neighboring microfibrils to a degree that might suggest they are individually unstable, although within collagen fibrils, they are so well ordered as to be crystalline.
A distinctive feature of collagen is the regular arrangement of amino acids in each of the three chains of these collagen subunits. The sequence often follows the pattern Gly-Pro-X or Gly-X-Hyp, where X may be any of various other amino acid residues. Proline or hydroxyproline constitute about 1/6 of the total sequence. With glycine accounting for the 1/3 of the sequence, this means approximately half of the collagen sequence is not glycine, proline or hydroxyproline, a fact often missed due to the distraction of the unusual GX1X2 character of collagen alpha-peptides. The high glycine content of collagen is important with respect to stabilization of the collagen helix as this allows the very close association of the collagen fibers within the molecule, facilitating hydrogen bonding and the formation of intermolecular cross-links. This kind of regular repetition and high glycine content is found in only a few other fibrous proteins, such as silk fibroin.
Collagen is not only a structural protein. Due to its key role in the determination of cell phenotype, cell adhesion, tissue regulation, and infrastructure, many sections of its non-proline-rich regions have cell or matrix association/regulation roles. The relatively high content of proline and hydroxyproline rings, with their geometrically constrained carboxyl and (secondary) amino groups, along with the rich abundance of glycine, accounts for the tendency of the individual polypeptide strands to form left-handed helices spontaneously, without any intrachain hydrogen bonding.
Because glycine is the smallest amino acid with no side chain, it plays a unique role in fibrous structural proteins. In collagen, Gly is required at every third position because the assembly of the triple helix puts this residue at the interior (axis) of the helix, where there is no space for a larger side group than glycine's single hydrogen atom. For the same reason, the rings of the Pro and Hyp must point outward. These two amino acids help stabilize the triple helix – Hyp even more so than Pro; a lower concentration of them is required in animals such as fish, whose body temperatures are lower than most warm-blooded animals. Lower proline and hydroxyproline contents are characteristic of cold-water, but not warm-water fish; the latter tend to have similar proline and hydroxyproline contents to mammals. The lower proline and hydroxyproline contents of cold-water fish and other poikilotherm animals leads to their collagen having a lower thermal stability than mammalian collagen. This lower thermal stability means that gelatin derived from fish collagen is not suitable for many food and industrial applications.
The tropocollagen subunits spontaneously self-assemble, with regularly staggered ends, into even larger arrays in the extracellular spaces of tissues. Additional assembly of fibrils is guided by fibroblasts, which deposit fully formed fibrils from fibripositors. In the fibrillar collagens, molecules are staggered to adjacent molecules by about 67 nm (a unit that is referred to as 'D' and changes depending upon the hydration state of the aggregate). In each D-period repeat of the microfibril, there is a part containing five molecules in cross-section, called the "overlap", and a part containing only four molecules, called the "gap". These overlap and gap regions are retained as microfibrils assemble into fibrils, and are thus viewable using electron microscopy. The triple helical tropocollagens in the microfibrils are arranged in a quasihexagonal packing pattern.
There is some covalent crosslinking within the triple helices and a variable amount of covalent crosslinking between tropocollagen helices forming well-organized aggregates (such as fibrils). Larger fibrillar bundles are formed with the aid of several different classes of proteins (including different collagen types), glycoproteins, and proteoglycans to form the different types of mature tissues from alternate combinations of the same key players. Collagen's insolubility was a barrier to the study of monomeric collagen until it was found that tropocollagen from young animals can be extracted because it is not yet fully crosslinked. However, advances in microscopy techniques (i.e. electron microscopy (EM) and atomic force microscopy (AFM)) and X-ray diffraction have enabled researchers to obtain increasingly detailed images of collagen structure in situ. These later advances are particularly important to better understanding the way in which collagen structure affects cell–cell and cell–matrix communication and how tissues are constructed in growth and repair and changed in development and disease. For example, using AFM–based nanoindentation it has been shown that a single collagen fibril is a heterogeneous material along its axial direction with significantly different mechanical properties in its gap and overlap regions, correlating with its different molecular organizations in these two regions.
Collagen fibrils/aggregates are arranged in different combinations and concentrations in various tissues to provide varying tissue properties. In bone, entire collagen triple helices lie in a parallel, staggered array. 40 nm gaps between the ends of the tropocollagen subunits (approximately equal to the gap region) probably serve as nucleation sites for the deposition of long, hard, fine crystals of the mineral component, which is hydroxylapatite (approximately) Ca10(OH)2(PO4)6. Type I collagen gives bone its tensile strength.
Associated disorders
Collagen-related diseases most commonly arise from genetic defects or nutritional deficiencies that affect the biosynthesis, assembly, posttranslational modification, secretion, or other processes involved in normal collagen production.
| Type | Notes | Gene(s) | Disorders |
| I | This is the most abundant collagen of the human body. It is present in scar tissue, the end product when tissue heals by repair. It is found in tendons, skin, artery walls, cornea, the endomysium surrounding muscle fibers, fibrocartilage, and the organic part of bones and teeth. | COL1A1, COL1A2 | Osteogenesis imperfecta, Ehlers–Danlos syndrome, infantile cortical hyperostosis a.k.a. Caffey's disease |
| II | Hyaline cartilage, makes up 50% of all cartilage protein. Vitreous humour of the eye. | COL2A1 | Collagenopathy, types II and XI |
| III | This is the collagen of granulation tissue and is produced quickly by young fibroblasts before the tougher type I collagen is synthesized. Reticular fiber. Also found in artery walls, skin, intestines and the uterus | COL3A1 | Ehlers–Danlos syndrome, Dupuytren's contracture |
| IV | Basal lamina; eye lens. Also serves as part of the filtration system in capillaries and the glomeruli of nephron in the kidney. | COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6 | Alport syndrome, Goodpasture's syndrome |
| V | Most interstitial tissue, assoc. with type I, associated with placenta | COL5A1, COL5A2, COL5A3 | Ehlers–Danlos syndrome (classical) |
| VI | Most interstitial tissue, assoc. with type I | COL6A1, COL6A2, COL6A3, COL6A5 | Ulrich myopathy, Bethlem myopathy, atopic dermatitis |
| VII | Forms anchoring fibrils in dermoepidermal junctions | COL7A1 | Epidermolysis bullosa dystrophica |
| VIII | Some endothelial cells | COL8A1, COL8A2 | Posterior polymorphous corneal dystrophy 2 |
| IX | FACIT collagen, cartilage, assoc. with type II and XI fibrils | COL9A1, COL9A2, COL9A3 | EDM2 and EDM3 |
| X | Hypertrophic and mineralizing cartilage | COL10A1 | Schmid metaphyseal dysplasia |
| XI | Cartilage | COL11A1, COL11A2 | Collagenopathy, types II and XI |
| XII | FACIT collagen, interacts with type I containing fibrils, decorin and glycosaminoglycans | COL12A1 | – |
| XIII | Transmembrane collagen, interacts with integrin a1b1, fibronectin and components of basement membranes like nidogen and perlecan. | COL13A1 | – |
| XIV | FACIT collagen, also known as undulin | COL14A1 | – |
| XV | – | COL15A1 | – |
| XVI | FACIT collagen | COL16A1 | – |
| XVII | Transmembrane collagen, also known as BP180, a 180 kDa protein | COL17A1 | Bullous pemphigoid and certain forms of junctional epidermolysis bullosa |
| XVIII | Source of endostatin | COL18A1 | – |
| XIX | FACIT collagen | COL19A1 | – |
| XX | – | COL20A1 | – |
| XXI | FACIT collagen | COL21A1 | – |
| XXII | FACIT collagen | COL22A1 | – |
| XXIII | MACIT collagen | COL23A1 | – |
| XXIV | – | COL24A1 | – |
| XXV | – | COL25A1 | – |
| XXVI | – | EMID2 | – |
| XXVII | – | COL27A1 | – |
| XXVIII | – | COL28A1 | – |
| XXIX | Epidermal collagen | COL29A1 | Atopic dermatitis |
In addition to the above-mentioned disorders, excessive deposition of collagen occurs in scleroderma.
Diseases
One thousand mutations have been identified in 12 out of more than 20 types of collagen. These mutations can lead to various diseases at the tissue level.
Osteogenesis imperfecta – Caused by a mutation in type 1 collagen, dominant autosomal disorder, results in weak bones and irregular connective tissue, some cases can be mild while others can be lethal. Mild cases have lowered levels of collagen type 1 while severe cases have structural defects in collagen.
Chondrodysplasias – Skeletal disorder believed to be caused by a mutation in type 2 collagen, further research is being conducted to confirm this.
Ehlers–Danlos syndrome – Thirteen different types of this disorder, which lead to deformities in connective tissue, are known. Some of the rarer types can be lethal, leading to the rupture of arteries. Each syndrome is caused by a different mutation. For example, the vascular type (vEDS) of this disorder is caused by a mutation in collagen type 3.
Alport syndrome – Can be passed on genetically, usually as X-linked dominant, but also as both an autosomal dominant and autosomal recessive disorder, those with the condition have problems with their kidneys and eyes, loss of hearing can also develop during the childhood or adolescent years.
Knobloch syndrome – Caused by a mutation in the COL18A1 gene that codes for the production of collagen XVIII. Patients present with protrusion of the brain tissue and degeneration of the retina; an individual who has family members with the disorder is at an increased risk of developing it themselves since there is a hereditary link.
Characteristics
Collagen is one of the long, fibrous structural proteins whose functions are quite different from those of globular proteins, such as enzymes. Tough bundles of collagen called collagen fibers are a major component of the extracellular matrix that supports most tissues and gives cells structure from the outside, but collagen is also found inside certain cells. Collagen has great tensile strength, and is the main component of fascia, cartilage, ligaments, tendons, bone and skin. Along with elastin and soft keratin, it is responsible for skin strength and elasticity, and its degradation leads to wrinkles that accompany aging. It strengthens blood vessels and plays a role in tissue development. It is present in the cornea and lens of the eye in crystalline form. It may be one of the most abundant proteins in the fossil record, given that it appears to fossilize frequently, even in bones from the Mesozoic and Paleozoic.
Uses
Collagen has a wide variety of applications, from food to medical. In the medical industry, it is used in cosmetic surgery and burn surgery. In the food sector, one use example is in casings for sausages.
If collagen is subject to sufficient denaturation, such as by heating, the three tropocollagen strands separate partially or completely into globular domains, containing a different secondary structure to the normal collagen polyproline II (PPII) of random coils. This process describes the formation of gelatin, which is used in many foods, including flavored gelatin desserts. Besides food, gelatin has been used in pharmaceutical, cosmetic, and photography industries. It is also used as a dietary supplement, and has been advertised as a potential remedy against the ageing process.
From the Greek for glue, kolla, the word collagen means "glue producer" and refers to the early process of boiling the skin and sinews of horses and other animals to obtain glue. Collagen adhesive was used by Egyptians about 4,000 years ago, and Native Americans used it in bows about 1,500 years ago. The oldest glue in the world, carbon-dated as more than 8,000 years old, was found to be collagen – used as a protective lining on rope baskets and embroidered fabrics, to hold utensils together, and in crisscross decorations on human skulls. Collagen normally converts to gelatin, but survived due to dry conditions. Animal glues are thermoplastic, softening again upon reheating, so they are still used in making musical instruments such as fine violins and guitars, which may have to be reopened for repairs – an application incompatible with tough, synthetic plastic adhesives, which are permanent. Animal sinews and skins, including leather, have been used to make useful articles for millennia.
Gelatin-resorcinol-formaldehyde glue (and with formaldehyde replaced by less-toxic pentanedial and ethanedial) has been used to repair experimental incisions in rabbit lungs.
=== 化粧品 ウシコラーゲンは、シワや皮膚の老化のエステティック矯正のための皮膚充填剤に広く使用されている。コラーゲンの繊維は大きすぎるため皮膚に浸透しないが、コラーゲンクリームも広く販売されている。コラーゲンサプリメントに関する研究のほとんどは、肯定的な研究結果から利益を得ることができる産業によって資金提供されている。
歴史
コラーゲンの分子構造や充填構造については、何十年にもわたる研究の間、科学者たちの頭を悩ませてきた。コラーゲンが分子レベルで規則正しい構造を持っているという最初の証拠は、1930年代半ばに発表された。その後、研究はコラーゲンモノマーのコンフォメーションに集中し、個々のペプチド鎖のコンフォメーションを正しく扱いながらも、いくつかの競合するモデルが生み出された。1955年にG. N. Ramachandranによって提唱された三重らせん状の「マドラス」モデルは、コラーゲンの四次構造の正確なモデルを提供した。このモデルは、20世紀後半により高い解像度の研究によって支持された。
コラーゲンの充填構造は、六角形であることは古くから知られているが、線維性コラーゲンタイプ以外では同程度には定義されていない。コラーゲンの単量体構造と同様に、コラーゲン分子のパッキング配列は「シート状」であるか、あるいはミクロフィブリル状であるという、いくつかの相反するモデルが提唱されている。腱、角膜、軟骨のコラーゲン線維のミクロフィブリル構造は、20世紀後半から21世紀初頭にかけて電子顕微鏡で直接撮影された。ラット尾腱のミクロフィブリル構造は、観察された構造に最も近いものとしてモデル化されたが、隣接するコラーゲン分子のトポロジカルな進行を単純化しすぎたため、ミクロフィブリルと呼ばれる不連続なD-周期的5量体配列の正しいコンフォメーションは予測できなかった。
こちらも参照
- コラーゲンハイブリダイジングペプチド, 変性コラーゲンに結合できるペプチド
- Hypermobility spectrum disorder/ja
- Metalloprotease inhibitor/ja
- オステオイド, 骨のコラーゲン含有成分
- Collagen loss/ja