Biguanide: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 4: | Line 4: | ||
{{short description|Chemical compound}} | {{short description|Chemical compound}} | ||
{{Pathnav|medication|diabetes medication|frame=1}} | {{Pathnav|medication|diabetes medication|frame=1}} | ||
{| class="wikitable sortable" style="width:100%" | |||
|- | |||
!一般名 | |||
!先発名 | |||
!class="unsortable"| 日本 | |||
!創薬/開発 | |||
!備考 | |||
|- | |||
|[[Metformin/ja]] | |||
|[https://sumitomo-pharma.jp/product/metgluco/ メトグルコ](Metgulco) ([https://pins.japic.or.jp/pdf/newPINS/00061908.pdf PI]) ([https://sumitomo-pharma.jp/product/metgluco/attachment/interv.html IF]) | |||
|販売 | |||
|[[住友ファーマ]] | |||
| | |||
|- | |||
|} | |||
{{chembox | {{chembox | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 49: | Line 64: | ||
'''Biguanide''' ({{IPAc-en|b|aɪ|ˈ|g|w|ɒ|n|aɪ|d}}) is the organic compound with the formula HN(C(NH)NH<sub>2</sub>)<sub>2</sub>. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to [[ammonia]] and [[urea]]. | '''Biguanide''' ({{IPAc-en|b|aɪ|ˈ|g|w|ɒ|n|aɪ|d}}) is the organic compound with the formula HN(C(NH)NH<sub>2</sub>)<sub>2</sub>. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to [[ammonia]] and [[urea]]. | ||
<!--T:3--> | |||
==Synthesis== | |||
Biguanide can be obtained from the reaction of [[dicyandiamide]] with [[ammonia]], via a [[pinner reaction|Pinner]]-type process. | Biguanide can be obtained from the reaction of [[dicyandiamide]] with [[ammonia]], via a [[pinner reaction|Pinner]]-type process. | ||
| Line 58: | Line 74: | ||
Biguanide was first synthesized by [[Bernhard Rathke]] in 1879. | Biguanide was first synthesized by [[Bernhard Rathke]] in 1879. | ||
<!--T:6--> | |||
==Biguanidine drugs== | |||
A variety of [[derivative (chemistry)|derivatives]] of biguanide are used as pharmaceutical drugs. | A variety of [[derivative (chemistry)|derivatives]] of biguanide are used as pharmaceutical drugs. | ||
<!--T:7--> | |||
===Antihyperglycemic agents=== | |||
The term "biguanidine" often refers specifically to a class of drugs that function as oral antihyperglycemic [[drug]]s used for [[diabetes mellitus]] or [[prediabetes]] treatment. | The term "biguanidine" often refers specifically to a class of drugs that function as oral antihyperglycemic [[drug]]s used for [[diabetes mellitus]] or [[prediabetes]] treatment. | ||
| Line 77: | Line 95: | ||
</gallery> | </gallery> | ||
<!--T:10--> | |||
====History==== | |||
{{details|metformin#History}} | {{details|metformin#History}} | ||
''[[Galega officinalis]]'' (French lilac) was used in diabetes treatment for centuries. In the 1920s, [[guanidine]] compounds were discovered in ''Galega'' extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, [[synthalin]] A and synthalin B, were used for diabetes treatment, but after the discovery of [[insulin]], their use declined. Biguanides were reintroduced into Type 2 [[diabetes]] treatment in the late 1950s. Initially [[phenformin]] was widely used, but its potential for sometimes fatal [[lactic acidosis]] resulted in its withdrawal from most pharmacopeias (in the U.S. in 1978). Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide. | ''[[Galega officinalis]]'' (French lilac) was used in diabetes treatment for centuries. In the 1920s, [[guanidine]] compounds were discovered in ''Galega'' extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, [[synthalin]] A and synthalin B, were used for diabetes treatment, but after the discovery of [[insulin]], their use declined. Biguanides were reintroduced into Type 2 [[diabetes]] treatment in the late 1950s. Initially [[phenformin]] was widely used, but its potential for sometimes fatal [[lactic acidosis]] resulted in its withdrawal from most pharmacopeias (in the U.S. in 1978). Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide. | ||
====Mechanism of action==== | <!--T:11--> | ||
====Mechanism of action==== | |||
The [[mechanism of action]] of biguanides is not fully understood, and many mechanisms have been proposed for metformin. | The [[mechanism of action]] of biguanides is not fully understood, and many mechanisms have been proposed for metformin. | ||
| Line 96: | Line 116: | ||
Biguanides have been shown to interact with copper, specifically in mitochondria, where they interfere with cell metabolism by chelating Copper in its 2+ oxidation state (Cu(II)). | Biguanides have been shown to interact with copper, specifically in mitochondria, where they interfere with cell metabolism by chelating Copper in its 2+ oxidation state (Cu(II)). | ||
====Side effects and toxicity==== | <!--T:16--> | ||
====Side effects and toxicity==== | |||
The most common side effect is [[diarrhea]] and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is [[lactic acidosis]], therefore metformin is contraindicated in advanced [[chronic kidney disease]]. Kidney function should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), [[hypoglycemia]] and other side effects are possible. | The most common side effect is [[diarrhea]] and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is [[lactic acidosis]], therefore metformin is contraindicated in advanced [[chronic kidney disease]]. Kidney function should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), [[hypoglycemia]] and other side effects are possible. | ||
<!--T:17--> | |||
===Antimalarial=== | |||
During WWII a British team led by [[Frank Rose (chemist)|Frank Rose]] discovered (see details there) that some biguanides are useful as [[antimalarial drug]]s. Much later it was demonstrated that they are prodrugs metabolised into active [[dihydrotriazine]] derivatives which, until recently, were believed to work by [[Dihydrofolate reductase inhibitor|inhibiting]] [[dihydrofolate reductase]]. Examples include: | During WWII a British team led by [[Frank Rose (chemist)|Frank Rose]] discovered (see details there) that some biguanides are useful as [[antimalarial drug]]s. Much later it was demonstrated that they are prodrugs metabolised into active [[dihydrotriazine]] derivatives which, until recently, were believed to work by [[Dihydrofolate reductase inhibitor|inhibiting]] [[dihydrofolate reductase]]. Examples include: | ||
* [[Proguanil]] (>[[cycloguanil]]) | * [[Proguanil]] (>[[cycloguanil]]) | ||
* [[Chlorproguanil]] | * [[Chlorproguanil]] | ||
<!--T:18--> | |||
===Disinfectants=== | |||
{{see also|Bisbiguanide}} | {{see also|Bisbiguanide}} | ||
The disinfectants [[chlorhexidine]], [[polyaminopropyl biguanide]] (PAPB), [[polihexanide]], and [[alexidine]] feature biguanide [[functional group]]s. | The disinfectants [[chlorhexidine]], [[polyaminopropyl biguanide]] (PAPB), [[polihexanide]], and [[alexidine]] feature biguanide [[functional group]]s. | ||
Latest revision as of 19:03, 23 April 2024
| 一般名 | 先発名 | 日本 | 創薬/開発 | 備考 |
|---|---|---|---|---|
| メトホルミン | メトグルコ(Metgulco) (PI) (IF) | 販売 | 住友ファーマ |

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Imidodicarbonimidic diamide | |
| Identifiers | |
3D model (JSmol)
|
|
| 507183 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 240093 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H7N5 | |
| Molar mass | 101.113 g·mol−1 |
| Acidity (pKa) | 3.07, 13.25 |
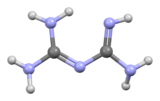
Biguanide (/baɪˈɡwɒnaɪd/) is the organic compound with the formula HN(C(NH)NH2)2. It is a colorless solid that dissolves in water to give highly basic solution. These solutions slowly hydrolyse to ammonia and urea.
Synthesis
Biguanide can be obtained from the reaction of dicyandiamide with ammonia, via a Pinner-type process.
Biguanide was first synthesized by Bernhard Rathke in 1879.
Biguanidine drugs
A variety of derivatives of biguanide are used as pharmaceutical drugs.
Antihyperglycemic agents
The term "biguanidine" often refers specifically to a class of drugs that function as oral antihyperglycemic drugs used for diabetes mellitus or prediabetes treatment.
Examples include:
- Metformin - widely used in treatment of diabetes mellitus type 2
- Phenformin - withdrawn from the market in most countries due to toxic effects
- Buformin - withdrawn from the market due to toxic effects
- bioactive biguanidines
-
Metformin, could be referred to as asymmetric dimethylbiguanidine
-
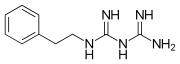
Phenformin. A phenethylated biguanidine.
History
Galega officinalis (French lilac) was used in diabetes treatment for centuries. In the 1920s, guanidine compounds were discovered in Galega extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, synthalin A and synthalin B, were used for diabetes treatment, but after the discovery of insulin, their use declined. Biguanides were reintroduced into Type 2 diabetes treatment in the late 1950s. Initially phenformin was widely used, but its potential for sometimes fatal lactic acidosis resulted in its withdrawal from most pharmacopeias (in the U.S. in 1978). Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide.
Mechanism of action
The mechanism of action of biguanides is not fully understood, and many mechanisms have been proposed for metformin.
Biguanides do not affect the output of insulin, unlike other hypoglycemic agents such as sulfonylureas and meglitinides. Therefore, they are effective in Type 2 diabetics; and in Type 1 diabetes when used in conjunction with insulin therapy.
Mainly used in Type II diabetes, metformin is considered to increase insulin sensitivity in vivo, resulting in reduced plasma glucose concentrations, increased glucose uptake, and decreased gluconeogenesis.
However, in hyperinsulinemia, biguanides can lower fasting levels of insulin in plasma. Their therapeutic uses derive from their tendency to reduce gluconeogenesis in the liver, and, as a result, reduce the level of glucose in the blood. Biguanides also tend to make the cells of the body more willing to absorb glucose already present in the bloodstream, and there again reducing the level of glucose in the plasma.
Biguanides have been shown to interact with copper, specifically in mitochondria, where they interfere with cell metabolism by chelating Copper in its 2+ oxidation state (Cu(II)).
Side effects and toxicity
The most common side effect is diarrhea and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is lactic acidosis, therefore metformin is contraindicated in advanced chronic kidney disease. Kidney function should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), hypoglycemia and other side effects are possible.
Antimalarial
During WWII a British team led by Frank Rose discovered (see details there) that some biguanides are useful as antimalarial drugs. Much later it was demonstrated that they are prodrugs metabolised into active dihydrotriazine derivatives which, until recently, were believed to work by inhibiting dihydrofolate reductase. Examples include:
Disinfectants
The disinfectants chlorhexidine, polyaminopropyl biguanide (PAPB), polihexanide, and alexidine feature biguanide functional groups.
| この記事は、クリエイティブ・コモンズ・表示・継承ライセンス3.0のもとで公表されたウィキペディアの項目Biguanide(13 January 2024編集記事参照)を素材として二次利用しています。 Item:Q21468 |