ビグアナイド
Biguanide/ja

| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Imidodicarbonimidic diamide | |
| Identifiers | |
3D model (JSmol)
|
|
| 507183 | |
| ChEBI | |
| ChemSpider | |
| EC Number |
|
| 240093 | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C2H7N5 | |
| Molar mass | 101.113 g·mol−1 |
| Acidity (pKa) | 3.07, 13.25 |
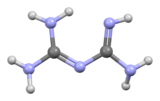
ビグアナイド(Biguanide、/b[invalid input: 'a&']ɡwɒnaɪd/)は、式HN(C(NH)NH2)2で表される有機化合物である。 無色の固体で、水に溶けて塩基性の高い溶液を作る。 これらの溶液はゆっくりと加水分解してアンモニアと尿素になる。
合成
ビグアニドはジシアンジアミドとアンモニアの反応から、ピナー型のプロセスを経て得ることができる。
ビグアナイドは1879年にBernhard Rathkeによって初めて合成された。
ビグアニジン薬物
ビグアニジンのさまざまな誘導体が薬物として使用されている。
抗高血糖薬
"ビグアニジン"という用語は、特に、糖尿病または糖尿病前症の治療に用いられる経口抗高血糖薬物として機能する一群の薬物を指すことが多い。
例えば、以下のようなものがある:
- bioactive biguanidines
-
Metformin, could be referred to as asymmetric dimethylbiguanidine
-
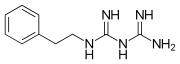
Phenformin. A phenethylated biguanidine.
History
Galega officinalis (French lilac) was used in diabetes treatment for centuries. In the 1920s, guanidine compounds were discovered in Galega extracts. Animal studies showed that these compounds lowered blood glucose levels. Some less toxic derivatives, synthalin A and synthalin B, were used for diabetes treatment, but after the discovery of insulin, their use declined. Biguanides were reintroduced into Type 2 diabetes treatment in the late 1950s. Initially phenformin was widely used, but its potential for sometimes fatal lactic acidosis resulted in its withdrawal from most pharmacopeias (in the U.S. in 1978). Metformin has a much better safety profile, and it is the principal biguanide drug used in pharmacotherapy worldwide.
Mechanism of action
The mechanism of action of biguanides is not fully understood, and many mechanisms have been proposed for metformin.
Biguanides do not affect the output of insulin, unlike other hypoglycemic agents such as sulfonylureas and meglitinides. Therefore, they are effective in Type 2 diabetics; and in Type 1 diabetes when used in conjunction with insulin therapy.
Mainly used in Type II diabetes, metformin is considered to increase insulin sensitivity in vivo, resulting in reduced plasma glucose concentrations, increased glucose uptake, and decreased gluconeogenesis.
However, in hyperinsulinemia, biguanides can lower fasting levels of insulin in plasma. Their therapeutic uses derive from their tendency to reduce gluconeogenesis in the liver, and, as a result, reduce the level of glucose in the blood. Biguanides also tend to make the cells of the body more willing to absorb glucose already present in the bloodstream, and there again reducing the level of glucose in the plasma.
Biguanides have been shown to interact with copper, specifically in mitochondria, where they interfere with cell metabolism by chelating Copper in its 2+ oxidation state (Cu(II)).
Side effects and toxicity
The most common side effect is diarrhea and dyspepsia, occurring in up to 30% of patients. The most important and serious side effect is lactic acidosis, therefore metformin is contraindicated in advanced chronic kidney disease. Kidney function should be assessed before starting metformin. Phenformin and buformin are more prone to cause acidosis than metformin; therefore they have been practically replaced by it. However, when metformin is combined with other drugs (combination therapy), hypoglycemia and other side effects are possible.
Antimalarial
During WWII a British team led by Frank Rose discovered (see details there) that some biguanides are useful as antimalarial drugs. Much later it was demonstrated that they are prodrugs metabolised into active dihydrotriazine derivatives which, until recently, were believed to work by inhibiting dihydrofolate reductase. Examples include:
消毒薬
消毒剤クロルヘキシジン、ポリアミノプロピルビグアニド(PAPB)、ポリヘキサニド、アレキシジンはビグアニド官能基を特徴とする。