Template:Infobox potassium
Jump to navigation
Jump to search
Page Template:Infobox element/styles.css has no content.
 Potassium pearls (in paraffin oil, ~5 mm each) | ||||||||||||||||||||||||||||||
| Potassium | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | silvery white, faint bluish-purple hue when exposed to air | |||||||||||||||||||||||||||||
| Standard atomic weight Ar°(K) | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| Potassium in the periodic table | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| Atomic number (Z) | 19 | |||||||||||||||||||||||||||||
| Electron configuration | [Ar] 4s1 | |||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 8, 1 | |||||||||||||||||||||||||||||
| Physical properties | ||||||||||||||||||||||||||||||
| Phase at STP | solid | |||||||||||||||||||||||||||||
| Melting point | 336.7 K (63.5 °C, 146.3 °F) | |||||||||||||||||||||||||||||
| Boiling point | 1030.793 K (757.643 °C, 1395.757 °F) | |||||||||||||||||||||||||||||
| Density (at 20° C) | 0.8590 g/cm3 | |||||||||||||||||||||||||||||
| when liquid (at m.p.) | 0.82948 g/cm3 | |||||||||||||||||||||||||||||
| Critical point | 2223 K, 16 MPa | |||||||||||||||||||||||||||||
| Heat of fusion | 2.33 kJ/mol | |||||||||||||||||||||||||||||
| Heat of vaporization | 76.9 kJ/mol | |||||||||||||||||||||||||||||
| Molar heat capacity | 29.6 J/(mol·K) | |||||||||||||||||||||||||||||
| Atomic properties | ||||||||||||||||||||||||||||||
| Oxidation states | −1, +1 (a strongly basic oxide) | |||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.82 | |||||||||||||||||||||||||||||
| Ionization energies |
| |||||||||||||||||||||||||||||
| Atomic radius | empirical: 227 pm | |||||||||||||||||||||||||||||
| Covalent radius | 203±12 pm | |||||||||||||||||||||||||||||
| Van der Waals radius | 275 pm | |||||||||||||||||||||||||||||
 | ||||||||||||||||||||||||||||||
| Other properties | ||||||||||||||||||||||||||||||
| Natural occurrence | primordial | |||||||||||||||||||||||||||||
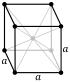
| Crystal structure | body-centered cubic (bcc) (cI2) | |||||||||||||||||||||||||||||
| Lattice constant | a = 532.69 pm (at 20 °C) | |||||||||||||||||||||||||||||
| Thermal expansion | 77.37×10−6/K (at 20 °C) | |||||||||||||||||||||||||||||
| Thermal conductivity | 102.5 W/(m⋅K) | |||||||||||||||||||||||||||||
| Electrical resistivity | 72 nΩ⋅m (at 20 °C) | |||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | |||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +20.8×10−6 cm3/mol (298 K) | |||||||||||||||||||||||||||||
| Young's modulus | 3.53 GPa | |||||||||||||||||||||||||||||
| Shear modulus | 1.3 GPa | |||||||||||||||||||||||||||||
| Bulk modulus | 3.1 GPa | |||||||||||||||||||||||||||||
| Speed of sound thin rod | 2000 m/s (at 20 °C) | |||||||||||||||||||||||||||||
| Mohs hardness | 0.4 | |||||||||||||||||||||||||||||
| Brinell hardness | 0.363 MPa | |||||||||||||||||||||||||||||
| CAS Number | 7440-09-7 | |||||||||||||||||||||||||||||
| History | ||||||||||||||||||||||||||||||
| Discovery and first isolation | Humphry Davy (1807) | |||||||||||||||||||||||||||||
| Symbol | "K": from New Latin kalium | |||||||||||||||||||||||||||||
| Isotopes of potassium | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| K · Potassium | ||
|---|---|---|
| Ar ← |
→ Ca | |
| indexes by PT (page) | ||
| Data sets read by {{Infobox element}} | |
|---|---|
| Name and identifiers | |
| Symbol etymology (11 non-trivial) | |
| Top image (caption, alt) | |
| Pronunciation | |
| Allotropes (overview) | |
| Group (overview) | |
| Period (overview) | |
| Block (overview) | |
| Natural occurrence | |
| Phase at STP | |
| Oxidation states | |
| Spectral lines image | |
| Electron configuration (cmt, ref) | |
| Isotopes | |
| Standard atomic weight | |
| most stable isotope | |
| Wikidata * | |
| * Not used in {{Infobox element}} (2023-01-01) See also {{Index of data sets}} · Cat:data sets (0) · (this table: ) | |
References
One of these is a named reference. It may be cited in the containing article as
- <ref name="CIAAW2013" /> for the source Atomic weights of the elements 2013 (from subtemplates used by {{Infobox element}})