Lumisterol
Jump to navigation
Jump to search
| |
| Names | |
|---|---|
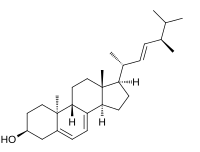
| IUPAC name
(22E)-9β,10α-Ergosta-5,7,22-trien-3β-ol
| |
| Systematic IUPAC name
(1R,3aR,7S,9aS,9bR,11aR)-1-[(2R,3E,5R)-5,6-Dimethylhept-3-en-2-yl]-9a,11a-dimethyl-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H44O | |
| Molar mass | 396.659 g·mol−1 |
Lumisterol is a compound that is part of the vitamin D family of steroid compounds. It is the (9β,10α) stereoisomer of ergosterol and was produced as a photochemical by-product in the preparation of vitamin D1, which was a mixture of vitamin D2 and lumisterol. Vitamin D2 can be formed from lumisterol by an electrocyclic ring opening and subsequent sigmatropic [1,7] hydride shift.
Lumisterol has an analog based on 7-dehydrocholesterol, known as lumisterol 3.
| この記事は、クリエイティブ・コモンズ・表示・継承ライセンス3.0のもとで公表されたウィキペディアの項目Lumisterol(4 May 2023編集記事参照)を素材として二次利用しています。 Item:Q22026 |