クエン酸カリウム
Potassium citrate/ja
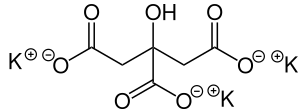
クエン酸カリウム(クエン酸三カリウムとも呼ばれる)はクエン酸のカリウム塩である。分子式K3C6H5O7で表される。白色の吸湿性の結晶性の粉末である。無臭で塩味がある。カリウムを38.28質量%含む。一水和物では、非常に吸湿性で潮解性である。
| |
| Names | |
|---|---|
| Preferred IUPAC name
Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| K3C6H5O7 | |
| Molar mass | 306.395 g/mol |
| Appearance | white powder hygroscopic |
| Odor | odorless |
| Density | 1.98 g/cm3 |
| Melting point | 180 °C (356 °F; 453 K) |
| Boiling point | 230 °C (446 °F; 503 K) |
| soluble | |
| Solubility | グリセリンに溶け、 エタノールに不溶である。 |
| Acidity (pKa) | 8.5 |
| Pharmacology | |
| A12BA02 (WHO) | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
170 mg/kg (IV, dog) 5400mg/kg (oral, rat) |
食品添加物として、クエン酸カリウムは酸味を調整するために使用され、Eナンバー E332として知られている。薬用としては、尿酸やシスチンに由来する腎結石の抑制に用いられることがある。
2020年には、米国で最も処方されている医薬品の297番目となり、処方件数は1 万件を超えた。
Synthesis
Potassium citrate can be synthesized by the neutralization of citric acid which is achieved by the addition of potassium bicarbonate, potassium carbonate or potassium hydroxide to it. The solution can then be filtered and the solvent can be evaporated till granulation.
Uses
Potassium citrate is rapidly absorbed when given by mouth, and is excreted in the urine. Since it is an alkaline salt, it is effective in reducing the pain and frequency of urination when these are caused by highly acidic urine. It is used for this purpose in dogs and cats, but is chiefly employed as a non-irritating diuretic.
Potassium citrate is an effective way to treat/manage arrhythmia, if the patient is hypokalemic.
It is widely used to treat urinary calculi (kidney stones), and is often used by patients with cystinuria. A systematic review showed a significant reduction in the incidence of stone formation RR 0.26, 95% CI 0.10 to 0.68.
It is also used as an alkalizing agent in the treatment of mild urinary tract infections, such as cystitis.
It is also used in many soft drinks as a buffering agent.
Frequently used in an aqueous solution with other potassium salts, it is a wet chemical fire suppressant that is particularly useful against kitchen fires. Its alkaline pH encourages saponification to insulate the fuel from oxidizing air, and the endothermic dehydration reaction absorbs heat energy to reduce temperatures.
Administration
Potassium citrate liquid is usually administered by mouth in a diluted aqueous solution, because of its somewhat caustic effect on the stomach lining, and the potential for other mild health hazards. Pill tablets also exist in normal, and extended-release formulations.
External links
- Tanner, G.A. "Potassium citrate improves renal function in rats with polycystic kidney disease". Journal of the American Society of Nephrology. Retrieved December 17, 2016.
| この記事は、クリエイティブ・コモンズ・表示・継承ライセンス3.0のもとで公表されたウィキペディアの項目Potassium citrate/ja(19 April 2024編集記事参照)を素材として二次利用しています。 Lua error in Module:Itemnumber at line 91: attempt to concatenate local 'qid' (a nil value). |