Translations:Discovery and development of dipeptidyl peptidase-4 inhibitors/46/en
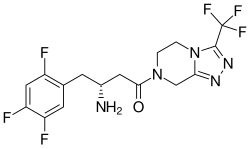
Sitagliptin (Januvia) has a novel structure with β-amino amide derivatives (Figure 7). Since sitagliptin has shown excellent selectivity and in vivo efficacy it urged researchers to inspect the new structure of DPP-4 inhibitors with appended β-amino acid moiety. Further studies are being developed to optimize these compounds for the treatment of diabetes. In October 2006 sitagliptin became the first DPP-4 inhibitor that got FDA approval for the treatment of type 2 diabetes. Crystallographic structure of sitagliptin along with molecular modeling has been used to continue the search for structurally diverse inhibitors. A new potent, selective and orally bioavailable DPP-4 inhibitor was discovered by replacing the central cyclohexylamine in sitagliptin with 3-aminopiperidine. A 2-pyridyl substitution was the initial SAR breakthrough since that group plays a significant role in potency and selectivity for DPP-4.